Orginal Research
2025
June
Volume : 13
Issue : 2
Role of conventional MRI and MR diffusion tensor imaging in cervical spine trauma: A comprehensive evaluation of the cervical cord
Shanmathi R, Kalaivani P, Bhuvaneswari KA, Murali N, Vasumathy S
Pdf Page Numbers :- 126-130
Shanmathi R1,*, Kalaivani P2, Bhuvaneswari KA2, Murali N2 and Vasumathy S2
1Department of Radiodiagnosis, Swamy Vivekanandha Medical College Hospital and Research Institute, Tiruchengode, Tamil Nadu 637205, India
2Department of Radiodiagnosis, Coimbatore Medical College, Coimbatore Medical College, Coimbatore, Tamil Nadu 641018, India
*Corresponding author: Dr. Shanmathi R, Assistant Professor, Department of Radiodiagnosis, Swamy Vivekanandha Medical College Hospital and Research Institute, Tiruchengode, Tamil Nadu 637205, India. Email: shanmathiram22@gmail.com
Received 3 December 2024; Revised 18 February 2025; Accepted 1 March 2025; Published 10 March 2025
Citation: Shanmathi R, Kalaivani P, Bhuvaneswari KA, Murali N, Vasumathy S. Role of conventional MRI and MR diffusion tensor imaging in cervical spine trauma: A comprehensive evaluation of the cervical cord. J Med Sci Res. 2025; 13(2):126-130. DOI: http://dx.doi.org/10.17727/JMSR.2024/13-22
Copyright: © 2025 Shanmathi R et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Cervical spine trauma was a critical clinical condition requiring accurate assessment for optimal management. This study aims to evaluate the role of Diffusion Tensor Imaging (DTI) and fiber tractography, alongside conventional MRI, in assessing cervical spinal cord injury (SCI). The objective was to analyse in vivo microstructural parameters—Fractional Anisotropy (FA) and Apparent Diffusion Coefficient (ADC)—using region of interest (ROI) analysis and to compare these parameters with conventional MRI findings for early detection of axonal injury.
Method: This hospital-based prospective observational study included 50 patients referred to the Department of Radio-Diagnosis for cervical spine MRI with clinical suspicion or confirmation of cervical spine trauma during 2022. Imaging was performed using a 1.5T Siemens Amira MR unit.
Results: The mean length of cord involvement was 3.39 cm on conventional MRI and 5.28 cm on DTI/MR tractography, a statistically significant difference. Patients with some or good clinical improvement had normal or increased ADC values, while those with decreased ADC showed no improvement. ADC was significantly reduced in cases of acute traumatic spinal cord injury (TSCI) and spinal cord haemorrhage. Although no strong correlation was found between FA values and clinical recovery, mean FA values were consistently lower in all cord pathologies, including swelling, contusion, haemorrhage, and transection.
Conclusion: DTI, with its quantitative indices, enhances the assessment of traumatic cervical spinal cord injury. Compared to conventional MRI, it provides additional valuable information. Incorporating DTI into routine imaging protocols may improve diagnostic accuracy and guide clinical management in spinal trauma.
Keywords: spinal cord injury; MRI; DTI; fractional anisotropy; apparent diffusion coefficient
Full Text
Introduction
Injuries to the cervical spine are a frequent contributor to significant morbidity and mortality in trauma patients. 6% of trauma patients have spine injuries of which >50% is contributed by cervical spine injury [1]. Jefferson found that injuries to the cervical spine involve two particular areas: C1-2 and C5-7. Meyer identified C2 and C5 as the two most common level of cervical spine injury. Injuries of the cervical spine produce neurological deficit in approximately 40% of patients [2]. Approximately 10% of traumatic cord injuries have no obvious radiographic evidence in conventional MRI. Early recognition, immobilization, preservation of spinal cord function and stabilization are the initial management of patients with cervical spine injuries with use of DTI to detect trauma related changes in spinal cord, in the armamentarium of cervical cord evaluation [3, 4]. Diffusion Tensor Imaging (DTI) is a non-invasive MRI technique that enables in vivo evaluation of spinal cord injury (SCI). It offers superior visualization of microstructural alterations compared to conventional MRI, as functional disruptions in SCI often occur before detectable structural changes appear on T2-weighted images.
However, DTI of the cervical cord was technically challenging due to low signal -to- noise of small volume of the cord, pulsation artifacts arising from CSF, cardiac and respiratory motion, magnetic susceptibility artifacts caused by adjacent bone[5-8] . But the ability of DTI and fibre tractography to diagnose subtle injuries aids in better therapeutic planning and optimal clinical outcome.
The study aimed to evaluate the role of DTI imaging and fiber tractography in SCI with in vivo micro structural parameter {Fractional Anisotropy (FA), Apparent Diffusion Coefficient (ADC)} using ROI and to compare DTI parameters (FA, ADC) with conventional MRI for early detection of axonal injury.
Materials and methods
It is a hospital based prospective observational study with sample size of 50 patients who present with neck pain, numbness, walking difficulty and neurological manifestation after SCI. The study was conducted in the year 2022 and it was approved by institutional ethics committee. MRI protocol acquisition technique was performed using a standard 1.5T MR unit (AMIRA SIEMENS). A standard cervical coil was used. Sequences used were T1W, T2W, STIR and Diffusion tensor imaging MRI (DTI, T2 SPACE). It was obtained using a single-shot echo planar imaging (EPI) sequence (b value = 1000 s/mm2) in diffusion encoding directions with scan time of approximately 10 min. The image was acquired in the sagittal plane with slice thickness of 5mm with no inter-slice gap, and a field of view (FOV) of 230mm, TR 3500ms, TE 108ms. The DTI images were analyzed using tractography software to generate color-coded FA and ADC maps in both axial and sagittal planes (Figure 1). To detect any abnormality in form of changes in the blue colour coded normally oriented craniocaudal cervical cord fibres. ROIs were carefully drawn to include only the cervical cord and not the surrounding CSF (Figure 2). ADC and FA values of ROIs-were obtained from C1 to T1 vertebral level. Collected data was presented in tables and diagrams. Frequency and percentages were calculated wherever applicable.
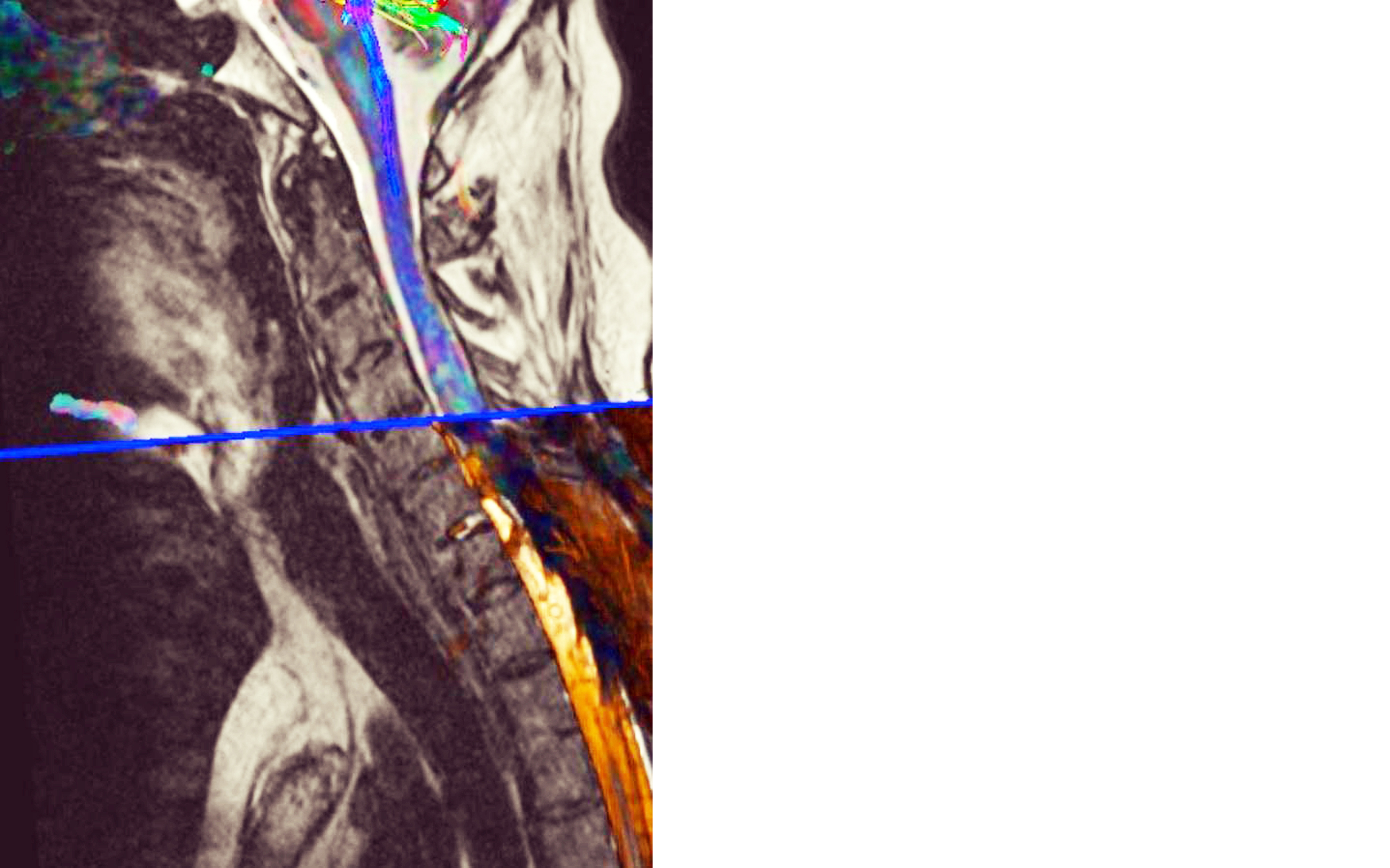
Figure 1: Tractography.
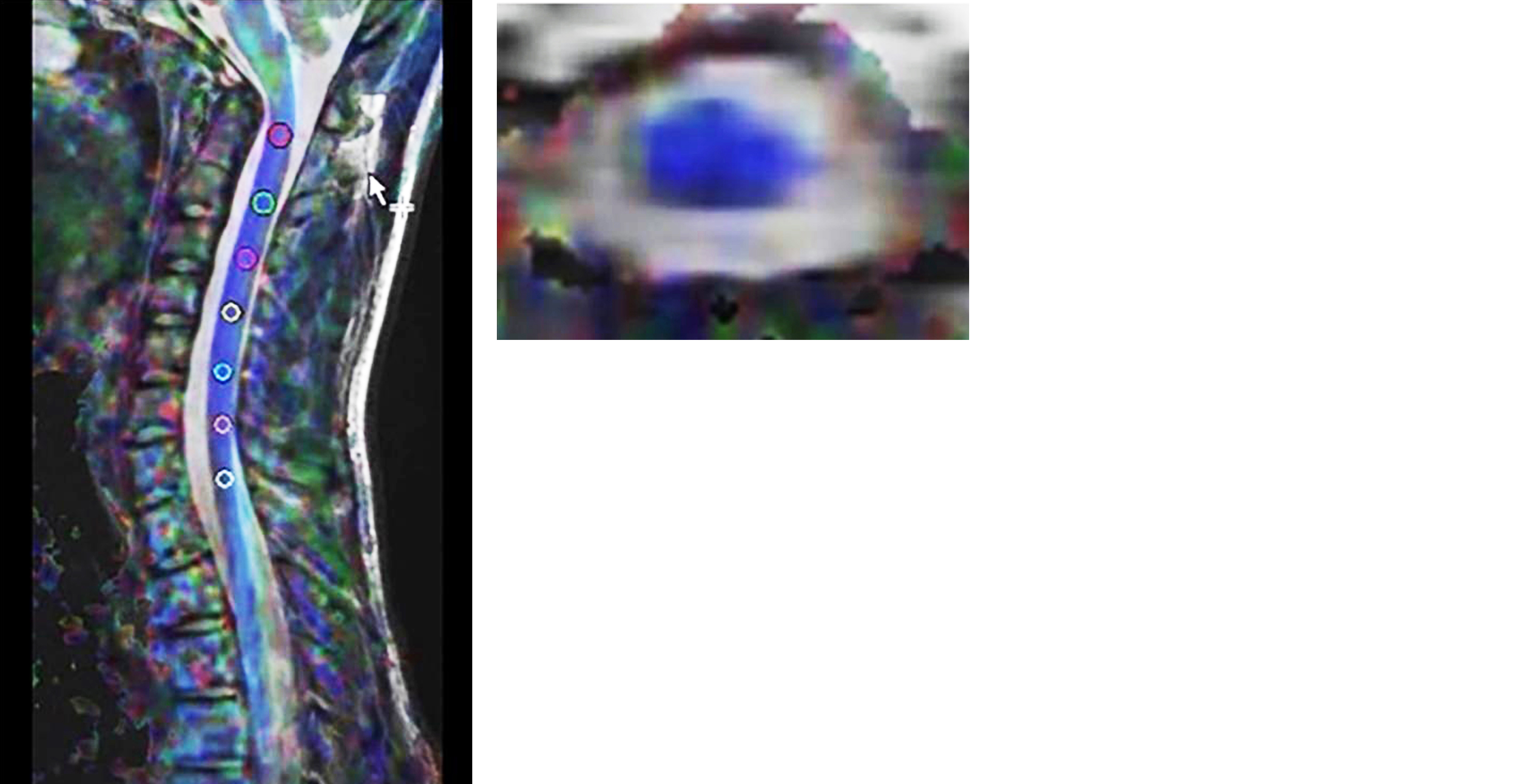
Figure 2: ROI marking.
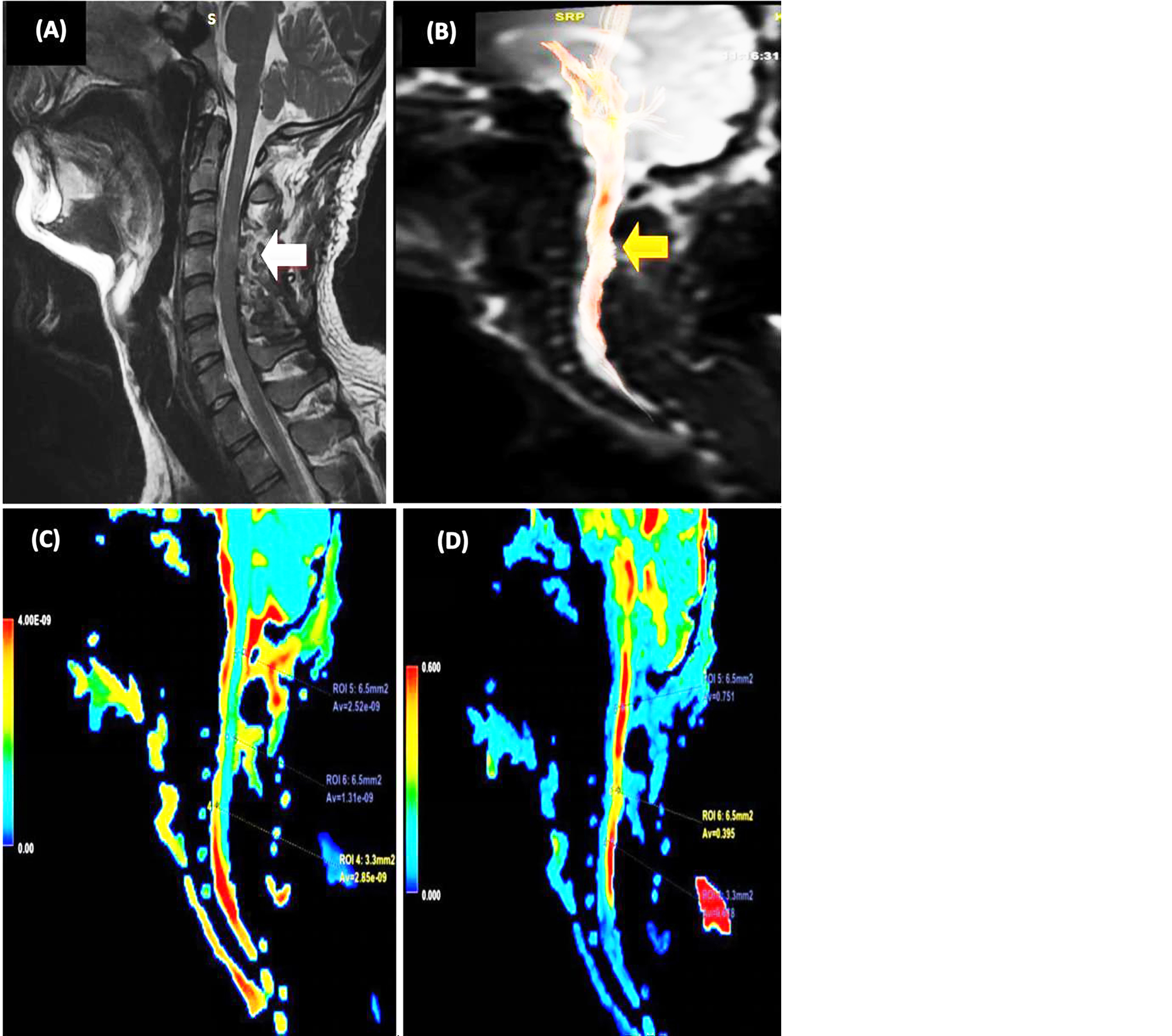
Figure 3: 22-year-old male with bike accident. (A) Abnormal spinal cord signal intensity at level of C4-C5. (B) Partially interrupted white matter tract of cervical spinal cord. (C) Color coded ADC map. (D) color coded FA map.
Results
Among 50 patients most of the patients were less than 30 years (34%) followed by 51-60 years (28%) and 41-50 years (22%) while rest were in 31- 40 years with male female ratio of 16:1 (Figure 4).
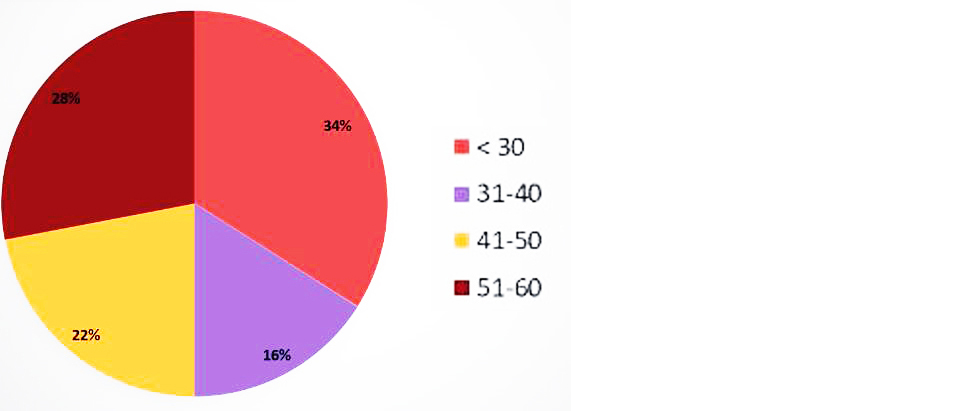
Figure 4: Age distribution.
Cord swelling was present in 7(14%), cord contusion in 40(80%), cord haemorrhage in 14(28%) and cord transection in only one patient (2%) (Figure 5).
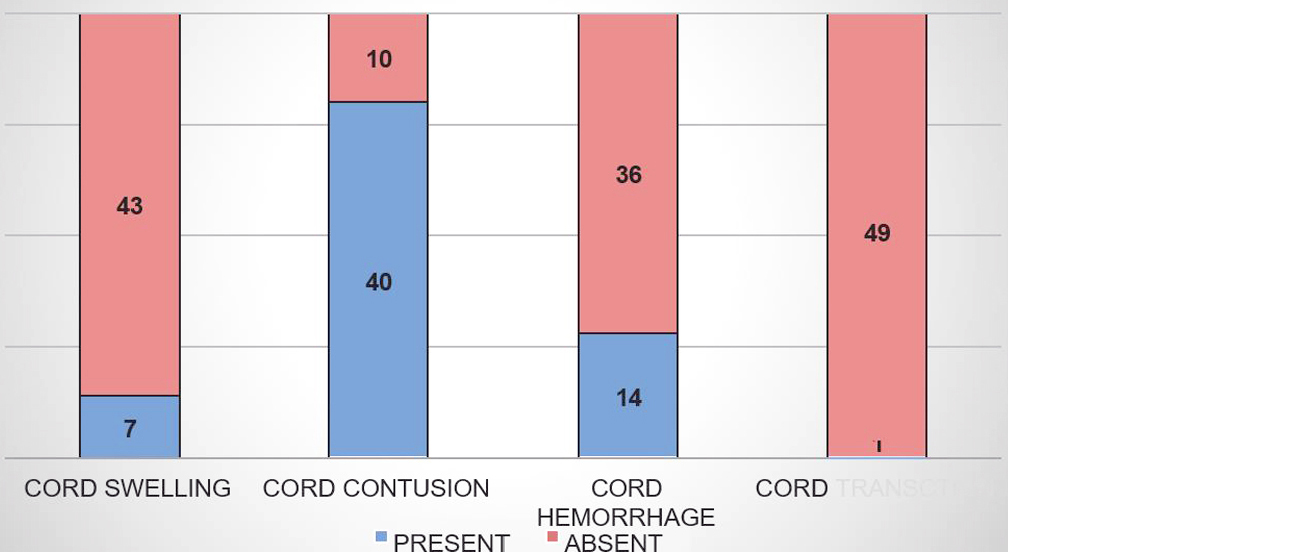
Figure 5: Cord changes.
In conventional MRI the mean cord length involved identified was 3.39 cm and while using DTI/MRT it was 5.28, thus patients with abnormalities in DTI had apparent cord abnormalities in conventional MRI and not the vice versa, making it clear that DTI superior to conventional MRI which was also statistically significant (Table 1).
Table 1: Injured cord level detected comparison.
|
Injured cord level detected
|
Conventional MRI
|
DTI/MR tractography
|
|
C1
|
0
|
7
|
|
C2
|
7
|
30
|
|
C3
|
25
|
46
|
|
C4
|
33
|
44
|
|
C5
|
33
|
41
|
|
C6
|
26
|
40
|
|
C7
|
23
|
34
|
|
D1
|
11
|
12
|
The mean ADC values were normal or higher in patients having cord swelling and transection (Table 2). While mean ADC was less in patient having cord contusion and haemorrhage in particular with significant p values. Similarly, the mean FA values are less in all cord changes like swelling, contusion, haemorrhage and transection (Table 3). But due to smaller sample size it was not statistically significant with a p value > 0.05. This decrease could be attributed to anisotropic diffusion restriction in traumatized spinal cord. FA values indirectly measure the extent of myelination. so, higher FA values indicate the integrity of spinal nerves.
Table 2: Cord changes vs mean apparent diffusion co efficient.
|
Cord changes
|
Mean ADC
|
P value
|
|
Present
|
Absent
|
|
|
Cord swelling
|
1.04
|
0.94
|
0.082
|
|
Cord contusion
|
0.93
|
1.07
|
0.016
|
|
Cord hemorrhage
|
0.83
|
1.01
|
0.001
|
|
Cord transection
|
1.17
|
0.95
|
0.197
|
Table 3: Cord changes vs mean fractional anisotropy.
|
Cord changes
|
Mean FA
|
P value
|
|
Present
|
Absent
|
|
Cord swelling
|
0.52
|
0.59
|
0.112
|
|
Cord contusion
|
0.5
|
0.54
|
0.387
|
|
Cord hemorrhage
|
0.51
|
0.54
|
0.461
|
|
Cord transection
|
0.51
|
0.53
|
0.826
|
Discussion
DTI helps to measure the water molecules displacement in tissue based of diffusion weighted imaging involving the principle of Brownian motion, i.e. free diffusion of water molecules within the extracellular space. Whenever there is restriction of water molecules, it is called diffusion restriction. It can be assessed quantitatively by measuring ADC.
Diffusion weighted images are based on the concept of isotropic diffusion in one single direction while in diffusion tensor imaging it is based on anisotropic diffusion of water molecules in different directions. Using 3T MRI up to 256 directions can be obtained. The axons in the nervous system permit dispersal of water molecules in parallel direction, rather than perpendicular directions. Common parameters used in diffusion tensor imaging are Fractional anisotropy (FA), Apparent diffusion coefficient (ADC), Regional anisotropy. Fractional anisotropy is used to delineate the degree of anisotropy in diffusion. It has a scalar value of zero to one. To say diffusion is isotropic the value should be close to zero. If the value is one, then it is anisotropic.
In MRI scanner X, Y, Z axes are not perfectly parallel to the central nervous system tract at each point. In Diffusion tensor imaging, images should be obtained in minimum of 6 directions usually at 12-24 directions. Pure ADC for each pixel is calculated from the images that are obtained in multiple directions known as Principal Eigen value. True axis of diffusion is called Eigen vector.
Using 3T MRI, water molecule will diffuse up to 256 directions. The direction of the maximum diffusivity is usually mapped using RGB (red, green, blue) colour channel [9].
DTI has been used in investigation of numerous pathologies that affects the brain. But its clinical application in spinal cord has been challenged by several difficulties because it is a very narrow structure, has a high elasticity and anatomically has a complex location. Other factors like CSF pulsation, motion artefacts and breathing related artefacts make it difficult to use as a routine sequence. So newer techniques like reduced field of view with oblique spin echo and OVS (outer volume suppression) have been used to reduce the limitation and to get better images. All these techniques provide a high image resolution not only in the cervical cord but also the entire spinal cord.
This study was done to evaluate the role of DTI imaging and fiber tractography in SCI with in vivo micro structural parameter {Fractional Anisotropy (FA), Apparent Diffusion Coefficient (ADC)} and also to compare DTI parameters (FA, ADC) with conventional MRI for early detection of axonal injury as hospital based prospective observational study in 50 patients with signs and symptoms of cervical spine trauma mainly traumatic cases with fracture c-spine /dislocation of c -spine on Conventional MR or CT. It can show the macroscopic orientation of fibers with elegant display of the disruption of tracts, which can hardly be seen on conventional MRI, allowing better delineation of damaged fiber tracts in the injured spinal cord. Wang, et al.[10] found that all patients with abnormalities in DTI had apparent cord abnormalities in conventional MRI. Conventional MRI is based mainly on signal intensity alteration for portrayal of abnormality [11]. Previous studies have shown conflicting results regarding the correlation between MRI findings and the severity of neurological injury. In accordance with our study as Shanmuganathan, et al. [12] who reported that ADC is significantly decreased in patients with acute TSCI and patients with spinal cord haemorrhage exhibiting the greatest decrease and Li XH, et al. [13] who also found that the mean value of ADC was significantly decreased at 24h and 72 h in the severely injured group. Hassen and El-Kholy [14] demonstrated a decrease in FA values in TSCI patients similar to our study. Regional measurements of FA values across five cord levels demonstrated a notable reduction at the site of cord injury.
In our study, due to the significant difference in the FA and ADC values at the site of trauma among patients as compared with controls.
Limitations: As this data was collected in tertiary care centre in limited patients with small sample size, do not precisely reflect the disease profile of the community. Admittedly, it is inadequate to provide conclusive data.
Conclusion
This study confirms that DTI, through its quantitative indices, can effectively detect microscopic structural changes in the spinal cord—even before the appearance of T2 signal changes on conventional MRI. FA values are more sensitive in detecting abnormalities at the injury site, while ADC values are more specific in cases of acute TSCI and spinal cord hemorrhage.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Rockwood CA, Green DP, Bucholz RW, Heckman JD, Court-Brown CM, et al. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p.1539–1947.
[2] Rockwood CA, Green DP, Bucholz RW, Heckman JD, Court-Brown CM, et al. Rockwood and Green’s Fractures in Adults. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p.1498–1541.
[3] Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ, et al. Intramedullary high signal intensity on T2‑weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 2001; 221:789–794.
[4] Fernández de Rota JJ, Meschian S, Fernández de Rota A, Urban V, Baron M, et al. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007; 6:17–22.
[5] Elshfiey I, Bilgen M, He R, Narayana PA. In vivo diffusion tensor imaging of rat spinal cord at 7 T. Magn Reson Imaging. 2002; 20:243–247.
[6] Agosta F, Benedetti B, Rocca MA, Valsasina P, Rovaris M, et al. Quantification of cervical cord pathology in primary progressive MS using diffusion tensor MRI. Neurology. 2005; 64:631–635.
[7] Demir A, Ries M, Moonen CTW, Vital JM, Arne B, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology. 2003; 229:37–43.
[8] Mamata H, Jolesz FA, Maier SE. Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis‑related changes. J Magn Reson Imaging. 2005; 22:38–43.
[9] Douek P, Turner R, Pekar J, Patronas N, Le Bihan D, et al. MR color mapping of myelin fiber orientation. J Comput Assist Tomogr. 1991; 15:923–929.
[10] Wang W, Qin W, Hao N, Wang Y, Zong G, et al. Diffusion tensor imaging in spinal cord compression. Acta Radiol. 2012; 53:921–928.
[11] Tator CH, Rowed DW, Schwartz ML. Sunnybrook cord injury scales for assessing neurological injury and neurological recovery in early management of acute spinal cord injury. In: Tator CH, editor. Early Management of Acute Spinal Cord Injury. New York: Raven Press; 1982. p.7–24.
[12] Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008; 29:655–659.
[13] Li XH, Wu F, Zhao F, Li-Huang S. Fractional anisotropy is a marker in early‑stage spinal cord injury. Brain Res. 2017; 1672:44–49.
[14] Hassen RZ, El-Kholy SF. Role of diffusion tensor imaging (DTI) in post‑traumatic spinal cord injury. Med J Cairo Univ. 2018; 86:4429–4433.
[15] Ranzenberger LR, Das JM, Snyder T. Diffusion Tensor Imaging. StatPearls Publishing; 2023.