Full Text
Introduction
Chronic liver disease can be described as a progressive deterioration of liver functions for duration of more than six months, associated with an unending cycle of inflammation, destruction and regeneration in the liver parenchyma which results in fibrosis or cirrhosis. It affects the functional ability of the liver to detoxify products of metabolism, synthesis of proteins, clotting factors and the excretion of bile [1]. Statistical data describe cirrhosis to be a common manifestation of chronic liver disease, affecting 0.27% of the general population. Among the cirrhotic population, 53.5% of individuals present with hepatitis C cirrhosis, alcoholic cirrhosis and nonalcoholic steatohepatitis - related cirrhosis [2].
The degree of elevations in aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) continue to be the mainstay for diagnoses [3]. However, it is desirable to note that fluid and electrolyte abnormalities, commonly seen amid those with chronic liver disease, may provide an emerging prognostic value. Hyponatremia, for instance, is common among those with end-stage liver disease (cirrhosis and portal hypertension) due to increased vasopressin activity and excessive renal retention of water. Patients presenting with ascites and hepatic encephalopathy often show dyselectrolytemia, commonly hyponatremia [4, 5]. Hyperkalemia, seen in 12-14% of patients with cirrhosis, is a common presentation among those with advanced cirrhosis, which could be a result of decreased urine output, potassium sparing diuretics or hemolysis in cirrhotic patients [6-8].
Literature identifies chloride as a predictor of mortality in chronic kidney disease, with little to no evidence of its study among the chronic liver disease population [9]. However, we consider chloride in this study, based on a hypothesis that dyselectrolytemia and kidney injury, both of which are common phenomena in chronic liver disease, may influence chloride levels to some extent. Creatinine, on the other hand, is often used as a parameter in predicting acute kidney injury (AKI) in the clinical setting. Studies show that those individuals with decompensated cirrhosis, are highly prone to develop AKI, which is of utmost clinical and prognostic relevance [10].
In our study, we hope to better understand the importance of dyselectrolytemia in liver dysfunction and the necessity of routine electrolyte tests performed in the clinical setting.
Method
A retrospective cohort study was conducted at PSG Institute of Medical Sciences and Research, Coimbatore between September 2021 to February 2022 following approval from the Institutional Ethical Committee (Ref. No.: PSG/IHEC/2022/Appr/Exp/012). Patients protected health information (PHI) were not collected nor documented in accordance with the Institutional Health Insurance Portability and Accountability Act (HIPAA) policy. Case sheets of all patients who presented to PSG hospitals between September 2021 to February 2022 who were diagnosed with chronic liver disease were reviewed and data of investigations conducted at 3 different time points, ‘at the time of admission’, ‘during hospitalization’ and ‘at the time of discharge’ was collected.
Out of a total of 600 reports, 492 were selected with respect to the inclusion and exclusion criteria. Only adult patients (19+ years) who presented with chronic liver disease whose signs and symptoms of liver disease persisted for a duration of more than six months due to alcoholic, post-infective, autoimmune and non-alcoholic steatohepatitis was evaluated. Patients who presented with pre-existing GIT malignancies, underlying renal diseases, acute liver failure and liver cell failure due to septicemia or endotoxemia other than primary liver cause were excluded from the study. The documented data was then subjected to statistical analysis and results were interpreted.
Results
The mean age of individuals in our study with chronic liver disease was 48.5 years. The sample consisted of individuals belonging to three age groups, ‘20-30 years’ consisting of 1 individual, ‘40-49 years’ consisting of 313 individuals and ‘50-59 years’ consisting of 178 individuals. Nearly 63.6% of individuals in our study were within 40-49 years of age which comprised the majority of the population. Meanwhile, 36.2% of individuals were within 50-59 years of age.
A total of 492 individuals were considered in the study. The study population consisted of 475 males and 17 females. The number of females was only 3.45 % of the total number of individuals considered in the study. The most common etiology in males was alcoholic liver disease which consisted of 267 individuals or 54.3% of our study population. The number of females was only 3.45 % of the total number of individuals considered in the study. All 17 females presented with nonalcoholic steatohepatitis with no incidence of alcoholic liver disease. The drastic gender based statistics in our study could be a result of the nature of etiologies that are commonly presented in the female population. Nevertheless, owing to the gender and age based statistics, we can safely say that our study would be more beneficial if extrapolated to the middle aged male population between the age groups of 40-49 years.
Sodium, potassium, chloride and creatinine were the four major parameters that were considered. In our study we have calculated the mean and standard deviations for each of these parameters with respect to each time point in hospitalization, that is ‘at the time of admission’, ‘during hospitalization’ and ‘at the time of discharge’. We have then studied the trends or variations of each of these electrolyte parameters across the different time points in hospitalization. Table 1 shows the mean and standard deviations of each parameter with respect to each time point of hospitalization. One sample t-test showed statistically significant variations in electrolyte levels (p<0.01) within each time point of hospitalization. Reference ranges for all electrolytes in this study have been written according to The American Board of Internal Medicine (ABIM) guidelines [11].
Table 1: Mean and Standard Deviation of Creatinine and Electrolytes with respect to time points in hospitalization.
|
Parameter
|
At the time of admission
|
During hospitalization
|
At the time of discharge
|
|
N
|
Mean
|
SD
|
N
|
Mean
|
SD
|
N
|
Mean
|
SD
|
|
Creatinine
|
167
|
1.679
|
1.4003
|
167
|
1.464
|
1.2041
|
167
|
1.33
|
1.0752
|
|
Sodium
|
164
|
131.17
|
6.65
|
164
|
131.85
|
5.687
|
163
|
132.64
|
5.644
|
|
Potassium
|
171
|
4.2397
|
1.23359
|
171
|
4.0643
|
0.74209
|
171
|
4.0715
|
0.64426
|
|
Chloride
|
163
|
99.64
|
9.9241
|
163
|
100.767
|
5.689
|
163
|
100.782
|
5.4753
|
Figure 1 shows the trend of creatinine across the different time points in hospitalization. The standard range of creatinine is between 0.7-1.3 mg/dL for men and 0.5-1.1 mg/dL for women. In our study, most patients with chronic liver disease presented with above normal creatinine levels at the time of admission. However, a decreasing trend of creatinine towards normalcy is noticed with the treatment of chronic liver disease.
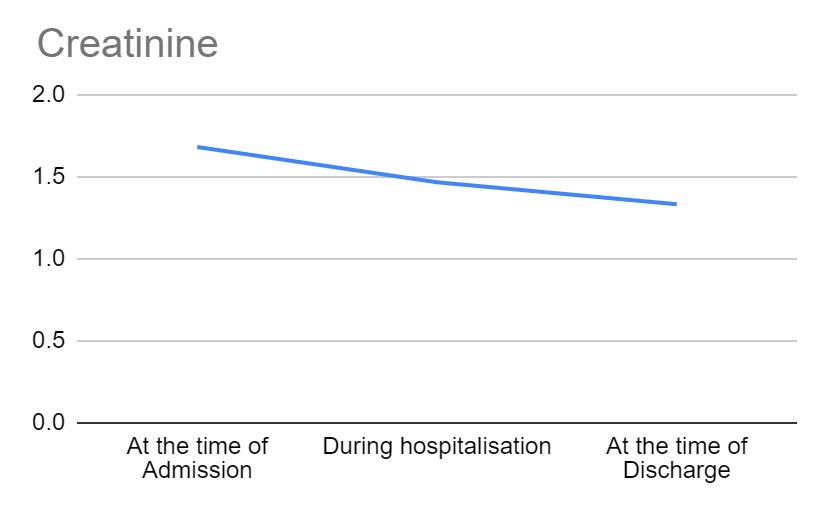
Figure 1: Variations in creatinine across different time points in hospitalization.
Figure 2 shows the trend of potassium across the different time points in hospitalization. Potassium values range between 3.5-5 mEq/L. In our study, potassium levels of all individuals at the time of admission was within the normal range. A decreasing tendency was seen from the time of admission to a time point during hospitalization, although no variations were seen beyond normalcy.
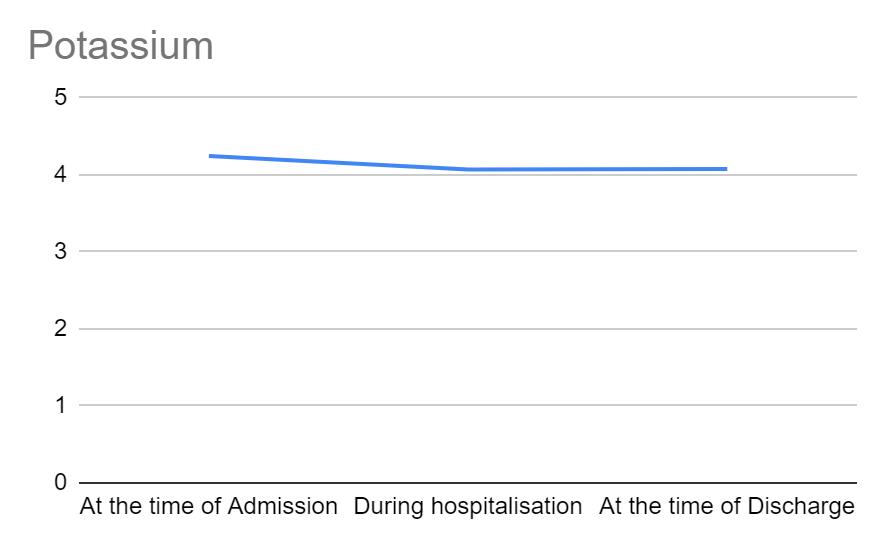
Figure 2: Variations in potassium across different time points in hospitalization.
Figure 3 shows the trend of sodium across the different time points in hospitalization. Sodium values range between 136-145 mEq/L. From the time of admission to discharge, sodium levels remained more or less similar with no deviation from normalcy.
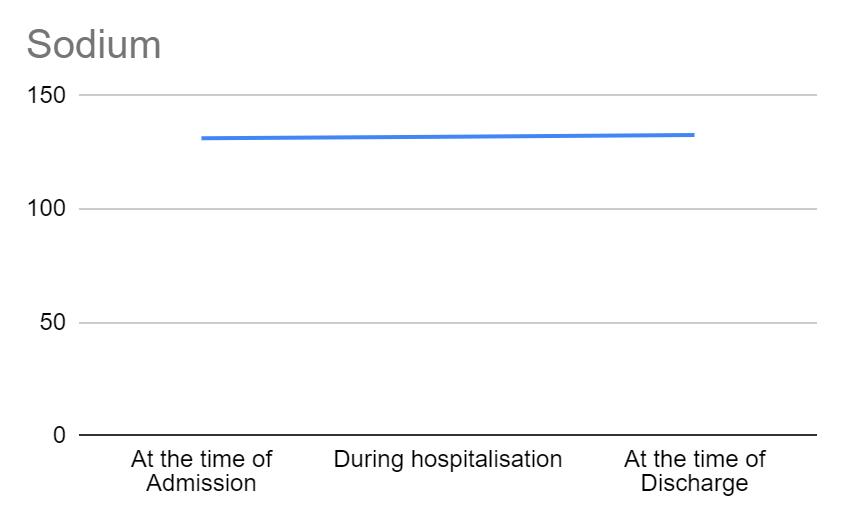
Figure 3: Variations in sodium across different time points in hospitalization. Figure 4 shows the trend of chloride across the different time points in hospitalization. Chloride values range between 98-106 mEq/L. From the time of admission to discharge, chloride levels remained more or less similar with no deviation from normalcy.
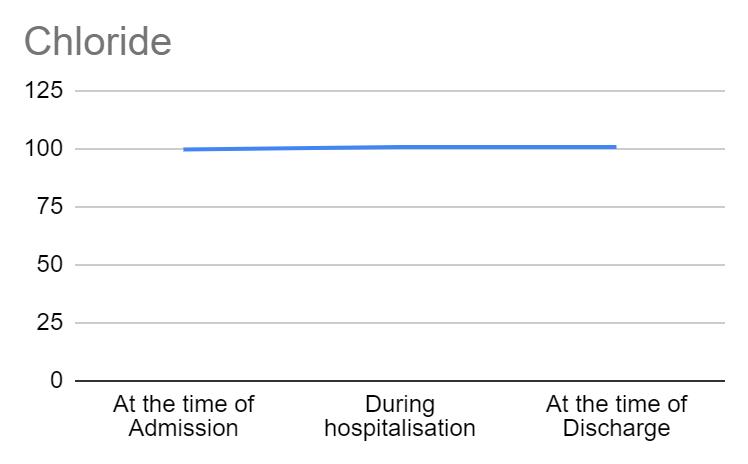
Figure 4: Variations in chloride across different time points in hospitalization.
Through these graphs, we can deduce that sodium and chloride showed no deviation from normalcy. Potassium showed a mild variation in the initial stages of hospitalization, but remained within normal limits and creatinine showed a decreasing trend towards normalcy.
Discussion
Individuals with chronic liver disease might exhibit a hypokalemic tendency which is likely due to decreased nutritional intake, vomiting, diuretic use or hyperaldosteronism seen in liver cirrhosis [12]. In our study, a decreasing trend in potassium levels was seen in the initial phase of hospitalization, however, no variations from normalcy was seen. Hepatic encephalopathy, a complication of chronic liver disease, is often aggravated by hypokalemia which leads to increased systemic ammonia levels [13], which may be contradictory to our study wherein any hepatic encephalopathy among our study population was associated with normokalemia and likely increase in systemic ammonia levels alone. Ullah et al showed that 22% of his study population were normokalemic despite having hepatic encephalopathy, which was consistent with our study [14]. Hyperkalemia may be associated with increased nutritional intake, decreased urine output or abnormal loss of potassium due to renal impairment and intake of potassium sparing diuretics [15]. In our study, potassium values were showing a downward shift within the reference range from being in a state of high normalcy. This may be a reflection of subtle variations in the homeostasis due to alcoholic liver disease, poor nutrition or of some infectious etiology. Moreover, creatinine levels showed a decreasing trend towards normalcy, suggestive of improving renal function which may or may not be associated with normal potassium levels.
In some cases of chronic liver disease, hyponatremia may be seen, probably due to renal impairment and retention of water [16]. In our study, creatinine levels suggest an improving renal function, which may or may not be related to the sodium values. Hypernatremia may occur in hepatic encephalopathy associated with increased water intake [17]. However, this phenomenon is rare and does not necessitate the need for regular sodium testing in individuals with chronic liver disease.
Little to no studies have documented the trends or complications of hyper/ hypochloremia in chronic liver disease. This corresponds to our study wherein chloride levels showed no major deviations from normalcy. Some studies indicate that chloride levels may vary in renal function, however, studying the trend of creatinine levels, a strong predictor of renal function, might render the regular estimation of chloride levels moot [9].
Creatinine is included in multiple cirrhosis prognostic criteria such as the Model for End-Stage Liver Disease (MELD) score, which takes into consideration 3 key components, namely, bilirubin, INR and creatinine levels [18]. Creatinine is a strong indicator of renal function, an increase in serum creatinine levels in chronic liver disease may be a result of portal hypertension causing altered renal perfusion due to splanchnic vasodilation, leading to hepatorenal syndrome, acute kidney injury, sepsis and hypovolemia. Some degree of renal impairment is often encountered in chronic liver disease [19]. In our study, the average creatinine levels were above the reference range at the time of admission. It showed a decreasing trend towards normalcy at the time of discharge, which indicates that the treatment of chronic liver disease is often associated with progressive resolution of renal function.
Some studies have drawn a correlation between age and the incidence of chronic liver diseases. Our study results are consistent with those studies. Estrogen is said to be a protective factor against inflammation and progression of liver impairment in healthy premenopausal women. Hence, the fall of estrogen levels in the post-menopausal age increases the severity of hepatic fibrosis, thus posing an increased risk for non-alcoholic fatty liver disease and rapid progression of liver diseases [20]. In our study, all women who presented with Non-alcoholic steatohepatitis belonged to the age group of 40-50 years which is consistent with the average menopausal age (45-55 years). Moreover, the variation in etiologies between the two genders in our study corroborates with literature that indicates an increased tendency for men to develop alcohol abuse disorders (14.1%) as compared to women (5.3%) [9].
Limitations: This study was performed in a tertiary care center among a small group of individuals. Regional and societal variations with respect to etiologies and biological response to medications was not taken into account. Hence, our data cannot be used to precisely reflect the disease profile of the community and the limited study population makes the data inconclusive to the larger community. A multicentric study with a larger study population might yield precise data.
Conclusion
During the course of hospitalization, sodium and chloride showed no deviation from normalcy, which may render routine sodium and chloride testing unnecessary in the future. Creatinine showed a decreasing trend towards normalcy, which indicates that an improvement in chronic liver failure may help in the improvement of renal function. Potassium showed a mild variation in the initial stages of hospitalization, but remained within normal limits. Potassium and creatinine are both strong indicators of renal function. Hence, in this study, we can conclude that the estimation of these parameters, especially creatinine may help prognosticate the status of liver disease through the status of renal function. Moreover, a decrease in renal and liver function has a potential to progress to multiorgan dysfunction. Nevertheless, further research is essential to better understand the association of these electrolyte parameters with chronic liver disease.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Sharma A, Nagalli S. Chronic Liver Disease. 2021 Jul 5. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Book.
[2] Acharya C, Bajaj JS. Gut microbiota and complications of liver disease. Gastroenterol Clin North Am. 2017; 46:155–169.
[3] Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018; 67:6–19.
[4] Bernardi M, Ricci C, Santi L. Hyponatremia in patients with cirrhosis of the liver. J Clin Med. 2014; 4:85–101.
[5] Praharaj DL, Anand AC. Clinical implications, evaluation, and management of hyponatremia in cirrhosis. J Clin Exp Hepatol. 2022; 12:575–594.
[6] Maiwall R, Kumar S, Sharma MK, Wani Z, Ozukum M, et al. Prevalence and prognostic significance of hyperkalemia in hospitalized patients with cirrhosis. J Gastroenterol Hepatol. 2016; 31:988–994.
[7] Nilsson E, Gasparini A, Ärnlöv J. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017; 245:277–284.
[8] Chen Y, Chang AR, DeMarco MA, Inker LA, Matsushita K, et al. Serum potassium, mortality, and kidney outcomes in the atherosclerosis risk in communities study. Mayo Clin Proc. 2016; 91:1403–1412.
[9] Khatri M, Zitovsky J, Lee D, Nayyar K, Fazzari M, et al. The association between serum chloride levels and chronic kidney disease progression: a cohort study. BMC Nephrol. 2020; 21:165.
[10] Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol. 2019; 25:3684–3703.
[11] American Board of Internal Medicine Laboratory Test Reference Ranges. Available from: https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf
[12] Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, et al. Hypokalemia: A clinical update. Endocr Connect. 2018; 7:R135–R146.
[13] Read AE, Laidlaw J, Haslam RM, Sherlock S. Neuropsychiatric complications following chlorothiazide therapy in patients with hepatic cirrhosis: Possible relation to hypokalaemia. Clin Sci. 1959; 18:409–423.
[14] Ullah H, Shabana H, Rady MA, Abdelsameea E, Youssef MI, et al. Hypokalemia as a responsible factor related with the severity of hepatic encephalopathy: a wide multination cross-sectional study. Ann Med Surg. 2023; 85:2427–2431.
[15] Cai JJ, Wang K, Jiang HQ, Han T. Characteristics, risk factors, and adverse outcomes of hyperkalemia in acute-on-chronic liver failure patients. Biomed Res Int. 2019; 2019:6025726.
[16] Gianotti RJ, Cardenas A. Hyponatremia and cirrhosis. Gastroenterol Rep. 2014; 2: 21–26.
[17] Hakimian S, Coukos J, Lui J, Guilarte-Walker Y, Mathews JP, et al. Hypernatremia in ICU patients with hepatic encephalopathy is associated with worse outcomes: 919. Official J Am College of Gastroenterol. 2017; 112:S515.
[18] Kim WR, Mannalithara A, Heimbach JK, Kamath PS, Asrani SK, et al. MELD 3.0: The model for end-stage liver disease updated for the modern era. Gastroenterol. 2021; 161:1887–1895.e4.
[19] Carrier P, Debette-Gratien M, Loustaud-Ratti V. Serum creatinine in cirrhotic patients: a cornerstone. AME Med J. 2018; 3:1–3.
[20] Brady CW. Liver disease in menopause. World J Gastroenterol. 2015; 21:7613.