Full Text
Introduction
Serous fluids such as pleural and peritoneal effusions are commonly produced in various non-neoplastic and neoplastic conditions. To identify the cause of effusion, these fluids are frequently subjected to cytopathological analysis. The sensitivity and specificity of cytopathological analysis of serous fluid in detecting malignancy ranges from 50% - 80% and 89% to 98% respectively [1].
Cytopathology reports consist of many descriptive terms which the clinicians find difficult to understand [2]. The international system (TIS) for reporting serous fluid cytopathology was developed and sponsored in 2020 by the international academy of cytology and American society of cytopathology [3, 4]. The aim of this newly adopted model was mainly to overcome clinical misunderstandings that paves way to undermined treatment decisions, by increasing interobserver agreement and thereby improves evidence based patient care and management.
The international system (TIS) for reporting serous fluid cytopathology has five diagnostic categories and they are as follows: (1) Non – diagnostic (ND), (2) Negative for malignancy (NFM), (3) Atypia of undetermined significance (AUS), (4) Suspicious for malignancy (SFM), and (5) Malignant (MAL).
This newly proposed diagnostic system has aimed at avoiding “uncertain” or “indeterminate” categories and has included AUS and SFM instead. Hence these categories will serve as a common language that bridges the gap between the clinician and the pathologist which ultimately improves better patient care based on ROM for each diagnostic criteria. In addition, TIS also helps in calculating the ROM for each diagnostic category. However, ROM varies from one laboratory to another and from one publisher to another based on availability of follow up tissue. Hence to overcome this overestimation of ROM due to selection bias, one can use the best ROM estimates in literature review. The most reliable implied ROM from literature calculated for individual category of TIS for reporting serous fluid cytopathology is as follows: (1) ND – 17% (+/- 8.9%), (2) NFM – 21% (+/- 0.3%), (3) AUS – 66% (+/- 10.6%), (4) SFM – 82% (+/- 4.8%), (5) MAL –99% (+/- 0.1%).
The Objectives of the study were (a) To classify the serous fluids cytologically into five categories using international system for reporting serous fluid cytology, (b) To adopt and to disseminate uniform system of reporting serous fluids and to avoid “uncertain” or “indeterminate” categories, (c) To evaluate diagnostic performance and to calculate risk of malignancy.
Materials and methods
This is a cross sectional study conducted between April 2020 to March 2022 at Vinayaka Mission’s Kirupananda Variyar Medical College and Hospitals, Salem, Tamil Nadu, India. All procedures performed in the current study were approved by the Institutional Ethical Committee.
This study included 400 cases of serous fluids which were received in the Department of Pathology during the study period. Non-random purposive sampling technique was used to select the sample size. Pleural, Peritoneal and Pericardial fluid obtained from patients of all age group and both sexes were included in the study. Fluids other than pleural, peritoneal and pericardial effusion and fluids of patients not willing to take part in the study were excluded from the study.
The standard handling of effusion samples in our laboratory consists of adequacy criteria being minimal 50ml followed by centrifugation and preparation of conventional smears from the sediment that are ethanol fixed for Papanicolaou staining and Hematoxylin and Eosin (H & E) staining, whereas air dried smears were stained with Giemsa stain and the remaining sample present were refrigerated at 2–8°C [5]. Immunocytochemistry (ICC) and cellblock preparations were reserved for cases that belonged to the AUS or SFM or MAL categories. Diagnostic routine in our department is carried out exclusively by all the pathologists posted in cytopathology division. Difficult cases were evaluated by the other senior pathologists as well. The parameters recorded from each cytology report included patients’ age, gender, and medical history, as well as each specimen’s site, nature and volume and other investigations like cell block or immunocytochemistry, if done.
Each cytology report was classified into one of the following categories, according to the TIS: (1) Non – diagnostic (ND) – Specimens with features such as: (a) Thick smears, (b) Factors such as blood or inflammation obscuring the morphology of cells of interest, (c) Distortion or rupture of smear during smearing techniques, (d) Totally hemolyzed samples, (e) Overstaining or incomplete staining, (f) Acellular Smears. (2) Negative for malignancy (NFM) – Specimens with cellular changes completely lacking evidence of mesothelial or non-mesothelial malignancy. (3) Atypia of undetermined significance (AUS) – Specimens showing limited cellular (nuclear) and/or architectural atypia. (4) Suspicious for malignancy (SFM) – Specimens showing features suspicious but not definitely diagnostic for malignancy. (5) Malignant (MAL) – Specimen with definitive findings and/or supportive studies indicating mesothelial or non-mesothelial malignancies (primary or secondary tumor).
Histopathology of tissue sample for the serous effusions received were analyzed and the corresponding blocks were subjected to immunohistochemical analysis wherever required. Clinical information and radiologic findings were also retrieved for the same from the electronic Medical Records Department of our Hospital. ROM assessment was calculated based on a combination of histology whenever available. Additional investigations like cell block, immunocytochemistry was done on samples reported as AUS, SFM and MAL.
Number of confirmed cases
ROM = ______________________________________
Total number of cases in the diagnostic category
|
Statistical Analysis
All the statistical analyses were done using statistical package for social services (SPSS - version 24).
Results
This study included 400 serous fluid effusion cases out of which, 140 (35%) were pleural fluid, 260 (65%) were peritoneal fluid and 0 pericardial fluid. Among the 140 pleural fluid effusions, 8 (5.7%) were reported as ND, 121 (86.4%) as NFM, 2 (1.4%) as AUS, 5(3.6%) as SFM and 4 (2.9%) as MAL. Among the 260 peritoneal fluid cases, 13 (5%), 226 (86.9%), 9 (3.5%), 9 (3.5%), and 3 (1.1%) were reported as ND, NFM, AUS, SFM and MAL respectively.
The age group of the pleural effusion and peritoneal fluid patients ranged between 14 years to 90 years, with a mean age of 52.08 years. The gender distribution among the pleural and peritoneal fluid cases was 177 (44.5%) females and 223 (55.5%) males, with a male to female ratio of 1.25:1. The age range for the pleural effusion is listed in (Table 1), for peritoneal effusion is listed in (Table 2) and gender distribution among pleural and peritoneal fluid is listed in (Table 3) respectively.
Table 1: Age range among various categories of pleural fluid.
|
TIS category
|
Age range in Years
|
Mean ± S.D
|
|
ND
|
25 – 78
|
47.1 ± 13.9
|
|
NFM
|
15 – 90
|
53.1 ± 16.2
|
|
AUS
|
43 – 50
|
46 ± 2.9
|
|
SFM
|
48 – 58
|
54 ± 3.4
|
|
MAL
|
37 - 80
|
59.7 ± 15.4
|
Table 2: Age range among various categories of peritoneal fluid.
|
TIS category
|
Age range in Years
|
Mean ± S.D
|
|
ND
|
26 – 88
|
54.2 ± 17.5
|
|
NFM
|
14 – 88
|
50.8 ± 14.0
|
|
AUS
|
44 – 86
|
54.2 ± 13.7
|
|
SFM
|
45 – 70
|
58.3 ± 9.6
|
|
MAL
|
56 - 75
|
64.3 ± 7.9
|
Table 3: Gender distribution among pleural and peritoneal fluid.
|
Fluid
|
Male
|
Female
|
M: F
|
|
Pleural
|
78
|
62
|
1.25:1
|
|
Peritoneal
|
145
|
115
|
1.26:1
|
Among the 400 serous effusion cases, tissue biopsy was available for 264/400 (66%) cases, out of which 11 cases (4.2%) belonged to the ND, 227 (85.98%) NFM, 8 (3.03%) AUS, 11(4.16%) SFM and 7 (2.65%) MAL. Status of histologic, clinical, and radiological correlation of pleural and pericardial effusion are mentioned in (Table 4) and (Table 5) respectively.
Table 4: Histological, clinical and radiological correlation for pleural fluid.
|
Cyto-diagnostic category
|
Total Number diagnosed by cytology
|
Histological correlation
|
Clinical and radiological correlation
|
|
Non-diagnostic (n=8)
|
08 (100%)
|
04 (50%)
|
04 (50%)
|
|
Negative for malignancy (n=121)
|
121(100%)
|
79 (65.2%)
|
42 (34.8%)
|
|
Atypia of undetermined significance (n=02)
|
02 (100%)
|
02 (100%)
|
0 (0%)
|
|
Suspicious for malignancy (n=05)
|
05 (100%)
|
04 (80%)
|
01 (20%)
|
|
Malignancy (n=04)
|
04 (100%)
|
04 (100%)
|
0 (0%)
|
|
Total (n=140)
|
140 (100%)
|
93 (66.4%)
|
47 (33.6%)
|
Table 5: Histological, clinical and radiological correlation for peritoneal fluid.
|
Cyto-diagnostic category
|
Total number diagnosed by cytology
|
Histological correlation
|
Clinical and radiological correlation
|
|
Non-diagnostic (n=13)
|
13 (100%)
|
07 (53.8%)
|
06 (46.2%)
|
|
Negative for malignancy (n=226)
|
226 (100%)
|
148 (65.5%)
|
78 (34.5%)
|
|
Atypia of undetermined significance (n=09)
|
09 (100%)
|
06 (66.7%)
|
03 33.3%)
|
|
Suspicious for malignancy (n=09)
|
09 (100%)
|
07 (77.8%)
|
02 (22.2%)
|
|
Malignancy (n=03)
|
03 (100%)
|
03 (100%)
|
0 (0%)
|
|
Total (n=260)
|
260 (100%)
|
171 (65.8%)
|
89 (34.2%)
|
ROM was calculated for the cases collected in this study are 0% for ND, 0.9% for NFM, 45.5% for AUS, 71.4% for SFM and 100% for MAL. The ROM for serous effusions including pleural and peritoneal fluid is given in the (Table 6).
Table 6: ROM for serous effusion cases.
|
Cyto-diagnostic category
|
ROM – pleural
|
ROM – peritoneal
|
ROM – total
|
|
Non-diagnostic
|
0/8 (0%)
|
0/13 (0%)
|
0/21 (0%)
|
|
Negative for malignancy
|
1/121(0.8%)
|
2/226 (0.9%)
|
3/347 (0.9%)
|
|
Atypia of undetermined significance
|
1/2 (50%)
|
4/9 (44.4%)
|
5/11 (45.5%)
|
|
Suspicious for malignancy
|
3/5 (60%)
|
7/9 (77.8%)
|
10/14 (71.4%)
|
|
Malignancy
|
4/4 (100%)
|
3/3 (100%)
|
7/7 (100%)
|
|
Total
|
9/140 (6.4%)
|
16/260 (6.1%)
|
25/400 (6.3%)
|
Diagnostic performance evaluation was performed for serous fluids received in our laboratory. Data’s available for ND, NFM and AUS were considered negative and SFM, MAL as positive results. For pleural fluid sensitivity was 80%, specificity 99.2%, PPV 88.8% and NPV 98.4%. For peritoneal fluid sensitivity was 83.3%, specificity 99.1%, PPV 83.3% and NPV 98.3%. A case of peritoneal effusion diagnosed as Suspicious for malignancy by cytology (Figure 1a) was confirmed as Deposit from adenocarcinoma ovary in the peritoneal tissue biopsy (Figure 1b).
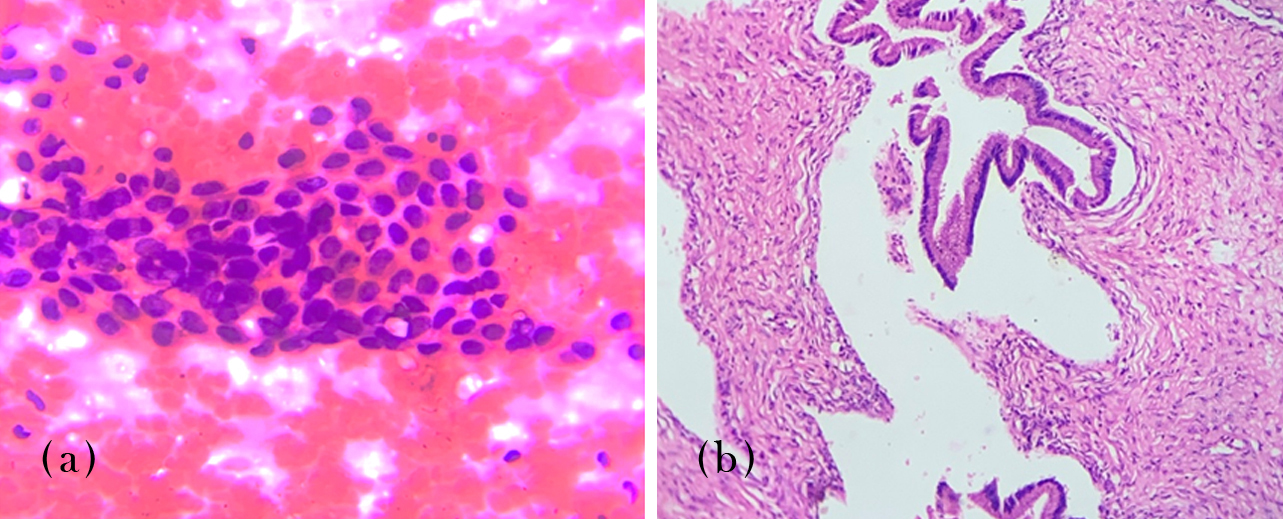
Figure 1: (a) Peritoneal fluid, suspicious for malignancy, 400x, H&E stain. (b) Peritoneal biopsy, deposit from adenocarcinoma ovary, 100x, H&E stain.
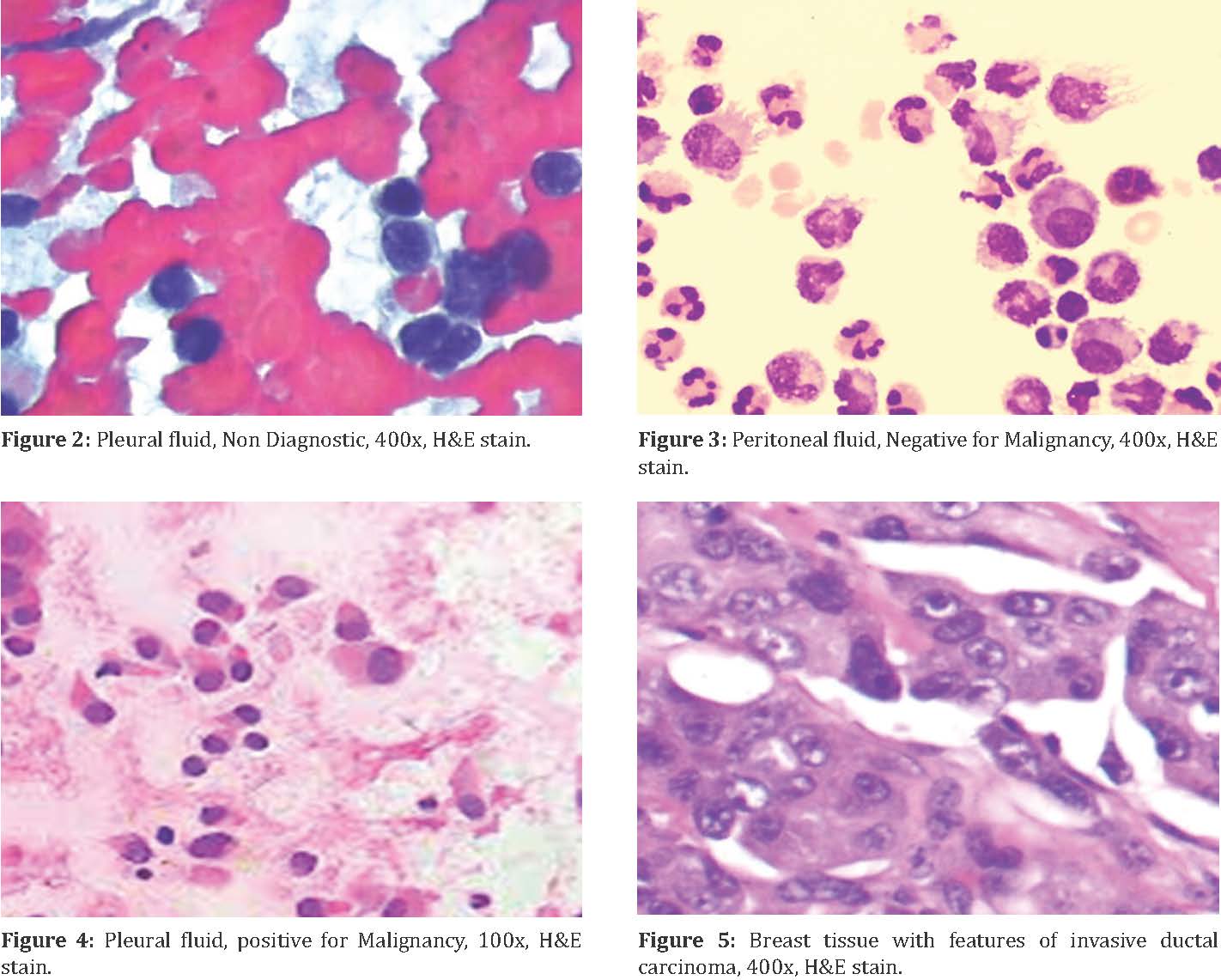
A case of Non diagnostic pleural effusion (Figure 2) with plenty of red blood cells and a case of peritoneal effusion diagnosed as negative for malignancy with few benign mesothelial cells (Figure 3).
A case of pleural effusion positive for malignancy (Figure 4) with the evidence of invasive ductal carcinoma in the breast (Figure 5).
Discussion
Serous fluids are commonly produced in various pathological processes and they are relatively easy to be collected. Hence, they are commonly submitted for cytopathological evaluation to identify the cause of the effusion. This study here concentrates on applying the TIS reporting system which has already established a strong support for its development by various qualified participants, to accurately identify the category of effusion, thereby find the cause [6, 7].
The age range in this study among the 140 pleural cases were 15 years to 90 years and 260 peritoneal effusion cases were 14 years to 88 years. In a study conducted by Alexandro Pergaris, the age range in pleural effusion patients were 11 years to 95 years and in peritoneal fluid cases were 16 years to 93 years [8]. Overall age range among all the 400 cases was 14 – 90 years with a Mean age of 52.08 years. In Zhu’s study the overall age range in the serous effusion patients were 9 years to 93 years with a mean age of 58.7 years [9]. The age range in both the studies are almost similar to that of our studies.
The gender distribution in our study among the 400 serous effusion cases showed a male preponderance with male (78 & 145 cases) to female (62 & 115 cases) ratio being 1.25:1 and 1.26:1 respectively in pleural and peritoneal effusion cases, whereas in a study conducted by Alexandro Pergaris, male preponderance (Male – 286 & Female – 242; M:F- 1.18:1) was noted among the pleural effusion cases but female preponderance (Male – 246 & Female – 254; M:F- 1:1.03) was noted among the peritoneal fluid cases. The overall male (222 cases) to female (178 cases) ratio being 1.2:1 in our study with a male preponderance, on the contrary, In Zhu’s and Sharma’s studies, there was a slight female preponderance with an M: F ratio of 1: 1.04 and 1:1.6 [9, 10]. The reason for male preponderance in our study may be attributed to less number of cases studied.
Among the 400 serous fluid effusion cases included in this study, 140 (35%) were pleural fluid, 260 (65%) were peritoneal fluid and 0 pericardial fluid and Kolte et al in their study categorized his cases into 366 (56.1%) cases of ascitic fluid followed by 262 (40.1%) cases of pleural fluid and 24 (3.8%) cases of pericardial fluid [11]. Out of the 140 (100%) pleural fluid cases, 8 (5.7%) were reported as ND, 121 (86.4%) as NFM, 2 (1.4%) as AUS, 5 (3.6%) as SFM and 4 (2.9%) as MAL, it was almost similar in a study conducted by Pinto et al and Jha et al with increase in MAL category [12, 13]. Among the 260 peritoneal fluid cases, 13 (5%), 226 (86.9%), 9 (3.5%), 9 (3.5%), and 3 (1.1%) were reported as ND, NFM, AUS, SFM and MAL respectively, on comparing our data with studies conducted by Rakheja et al and Straccia et al, malignancy cases were high in their studies comparatively [14, 15]. Overall TIS categorization of all the 400 fluid effusions were, 21 cases (5.25%) were reported as ND, 347 (86.75%) as NFM, 11 (2.75%) as AUS, 14 (3.5%) as SFM and 7 (1.75%) as MAL. Among the 400 serous effusion cases, tissue biopsy was available for 264/400 (66%) cases, out of which 11 cases (4.2%) belonged to the ND, 227 (85.98%) NFM, 8 (3.03%) AUS, 11(4.16%) SFM and 7 (2.65%) MAL. Similar to the pleural and peritoneal cases, the overall statistics also when compared to a study conducted by Valerio et al shows increase in malignancy cases [16]. Since others studied were conducted in the cancer centers, number of malignancy cases diagnosed in our study appears to be low.
Most common malignancies seen in pleural fluid were metastatic carcinoma of breast in females and adenocarcinoma GIT in males, whereas in peritoneal fluid, metastatic deposit of ovarian carcinoma in females and adenocarcinoma colon in males. The same was seen in a study conducted by Pergaris with ovaries, stomach and breast being the first three common malignancies with serous cavity metastasis [8].
ROM calculated for the cases collected in this study are 0% for ND, 0.9% for NFM, 45.5% for AUS, 71.4% for SFM and 100% for MAL, when compared to a study conducted by Farahani et al., Chandra A et al and Rakheja et al the ROM was 17%, 22%, 66%, 82% and 99% for the TIS categories ND, NFM, AUS, SFM and MAL respectively [17, 18]. The main discordance was seen among the ND and NFM categories, which mainly was because of loss of clinical follow up and lack of tissue biopsy or radiological details. However, the other categories such as AUS, SFM and MAL were almost close to the other studies conducted by Pergaris et al and Lobo et al [8, 19].
According to retrospective study conducted by Zhu for reporting serous fluid, the sensitivity was 86.5%, specificity was 99.4%, PPV was 99.8%, NPV was 68.3% for pleural effusion and the sensitivity was 88.4%, specificity was 99.6%, PPV was 99.9%, NPV was 70.3% for peritoneal effusion. Pergaris et al in his study evaluated laboratory diagnostic performance and showed that sensitivity was 75.9%, specificity 99.7%, PPV 98.75% and NPV 93.6% for pleural effusion and 80% sensitivity, 99.3% specificity, 98.5% PPV and 89.6% NPV [8]. The results of both the studies mentioned above are comparable with the results of present study.
Limitations of the study were smaller sample size, ancillary studies on wide number of cases were not performed due to lack of logistic support, and less number of malignancy cases were included in the study as ours is not an oncology center.
Conclusion
The international system (TIS) for reporting serous fluid cytopathology is very easy to employ and gives high accuracy with clear diagnostic criteria for each category. This system also makes it easy to communicate with the clinicians by employing simple terminologies. Classification of the “uncertain” category into AUS and SFM has further made it easy to diagnose and plan the treatment strategy.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Hou T, Landon G, Stewart J, Roy-Chowdhuri S. The value of a tiered cytology diagnostic reporting system in assessing the risk of malignancy in indeterminate serous effusions. Cancer Cytopathol. 2021; 129:75–82.
[2] Pinto D, Chandra A, Crothers BA, Kurtycz DFI, Schmitt F. The international system for reporting serous fluid cytopathology-diagnostic categories and clinical management. J Am Soc Cytopathol. 2020; 9:469–477.
[3] Pinto D, Chandra A, Schmitt F. The international system for reporting serous fluid cytopathology: How to incorporate molecular data in cytopathology reports. J Molec Pathol. 2021; 2:66–76.
[4] Ahmed MD, Wang H. Cytopathology in focus: Serous fluid cytopathology – Recent progress and Yale’s Experience. CAP Today, 2023.
[5] Srinivasan R, Rekhi B, Rajwanshi A, Pathuthara S, Mathur S, et al. Indian academy of cytologists guidelines for collection, preparation, interpretation, and reporting of serous effusion fluid samples. J Cytol. 2020; 37:1–11.
[6] Shidham VB, Layfield LJ. Approach to diagnostic cytopathology of serous effusions. Cytojournal. 2021; 18:32.
[7] Bussche CJV, Crothers B, Chandra A, Schmitt F, Kurtycz DFI. The international system for reporting serous fluid cytopathology: The initial project survey. Cytopathol. 2023; 34:191–197.
[8] Pergaris A, Stefanou D, Keramari P, Sousouris S, Kavantzas N, et al. Application of the international system for reporting serous fluid cytopathology with cytohistological correlation and risk of malignancy assessment. Diagnostics. 2021; 11:2223.
[9] Zhu YL, Ren WH, Wang Q, Jin HZ, Guo YY, et al. A retrospective analysis of serous effusions based on the newly proposed international system for reporting serous fluid cytopathology: a report of 3633 cases in an oncological center. Diagn Pathol. 2022; 17:56.
[10] Sharma N, Dubey K, Gurubasavaraj H, Hiremath SS. Peritoneal fluid analysis clinicocytological study. Int J Contemp Med Res. 2019; 6:112–116.
[11] Kolte S, Zaheer S, Aden D, Ranga S. Application of the international system for reporting serous fluid cytopathology on reporting various body fluids; experience of a tertiary care hospital. Cytojournal. 2022; 19:52.
[12] Pinto D, Cruz E, Branco D, Linares C, Carvalho C, et al. Cytohistological correlation in pleural effusions based on the international system for reporting serous fluid cytopathology. Diagnostics. 2021; 11:1126.
[13] Jha S, Sethy M, Adhya AK. Application of the international system for reporting serous fluid cytopathology in routine reporting of pleural effusion and assessment of the risk of malignancy. Diagn Cytopathol. 2021; 49:1089–1098.
[14] Rakheja G, Singh M, Priyadarshnee B, Marimuthu B, Dhar L, et al. Categorisation of peritoneal serous effusions using the international system for reporting serous fluid cytopathology-A study on gynaecological samples. Cytopathol. 2023; 34:138–145.
[15] Straccia P, Chiappetta M, Magnini D, Cancellieri A. Application of the international system for reporting serous fluid cytopathology (TIS): A retrospective institutional study. Cytopathol. 2022; 33:305–311.
[16] Valerio E, Nunes W, Cardoso J, Santos A, Bovolim G, et al. A 2-year retrospective study on pleural effusions: A cancer centre experience. Cytopathol. 2019; 30:607–613.
[17] Farahani SJ, Baloch Z. Are we ready to develop a tiered scheme for the effusion cytology? A comprehensive review and analysis of the literature. Diagn Cytopathol. 2019; 47:1145–1159.
[18] Chandra A, Crothers B, Kurtycz D, Schmitt F. Announcement: The international system for reporting serous fluid cytopathology. Acta Cytol. 2019; 63:349–351.
[19] Lobo C, Costa J, Petronilho S, Monteiro P, Leça L, et al. Cytohistological correlation in serous effusions using the newly proposed international system for reporting serous fluid cytopathology: Experience of an oncological center. Diagn Cytopathol. 2021; 49:596–605.