Full Text
Introduction
Tubal factor infertility is among the leading causes of female infertility, accounting for approximately 30–40% of cases. The fallopian tubes play a critical role in ovum transport and fertilization, and any disruption can significantly affect conception outcomes.
MR-HSG, a recent advancement, allows detailed uterine and tubal evaluation without ionising radiation. In addition to detecting tubal occlusions, it provides high-resolution imaging of pelvic structures, identifying concurrent pathologies such as fibroids, adenomyosis, and congenital uterine anomalies.
Recent research continues to support the shift toward non-invasive imaging modalities in infertility diagnostics. Studies by Meena et al. [1] and Yadav et al. [2] highlighted the superiority of Magnetic resonance hysterosalpingography (MR-HSG) over conventional hysterosalpingography in delineating uterine and adnexal abnormalities, noting its greater sensitivity and patient comfort. In a multicentric trial conducted in 2023, Sharma et al. [3] observed that MR-HSG demonstrated comparable diagnostic performance to laparoscopy, particularly in detecting peritubal adhesions and endometrial abnormalities. Moreover, the integration of AI-assisted imaging interpretation, as investigated by Li et al. [4], has significantly enhanced MR-HSG's accuracy, reducing interpretation variability. These advancements position MR-HSG not only as a viable alternative but potentially a preferred initial diagnostic tool in infertility workups [5].
This study aims to assess the diagnostic accuracy of MR-HSG compared to C-HSG in evaluating tubal patency and infertility-related abnormalities.
Materials and methods
A prospective observational study was conducted in the Department of Radio Diagnosis, Coimbatore Medical College Hospital, between March 2023 and February 2024. The study was approved by the Institutional Ethics Committee, and informed consent was obtained from all participants. A total of 30 women aged between 18 and 45 years, presenting with primary or secondary infertility, were enrolled in the study. Patients were selected based on predefined inclusion and exclusion criteria.
Inclusion criteria: Women aged 18–45 years presenting with unexplained infertility, normal baseline hormonal and ovulatory status, and no history of prior tubal surgery were included in the study.
Exclusion criteria: Patients with active pelvic infections, contraindications to MRI (such as metallic implants or claustrophobia), or confirmed/suspected pregnancy were excluded.
The study used a 1.5 Tesla MRI scanner to perform MR-HSG and limited pelvic MRI. A specialized imaging protocol was followed to maximize diagnostic yield while ensuring patient safety. The prior informed and detailed consent of the patient is obtained before the procedure. The patients was given oral mefenamic acid three times a day and a course of antibiotics (combination of ofloxacin and metronidazole) as premedication starting on the day before and continued two days post procedure. The patient’s bladder is made empty before the procedure. This is to ensure the patient comfort and ease of the procedure. The patient is asked to lie in a lithotomy position. Strict asepsis is achieved by using povidone iodine over the external genitalia and the routine surgical asepsis methods are followed with sterile aprons and autoclaved instruments. Initial examination of the external genital area and the vaginal introitus is conducted to look for the uterus size and position of the uterus.
The female vagina is stretched using a speculum and further cleaning of the cervix up to the external OS is done by the same antiseptic solution. Later 5 F micro catheter which is made up of rubber or silicon is inserted into the uterus and the bulb is inflated with the distilled water.
Later the instruments like valsellum and speculum are removed, and the patient is moved to the MRI room to take a series of images in various sequences with a 1.5 T MRI.
After acquisition of preliminary localizing sequences, the following sequences are obtained.
1. T2 weighted axial (TR/TE 7120/90; Flip angle 90 degree; section thickness 5 mm).
2. T2 weighted Coronal.
3. T2 weighted Saggital.
4. T1 weighted axial (TR/TE 740/13; Flip angle 90 degree; section thickness 5 mm).
5. T1 Cube Coronal – 5 phases each phase scanning for 15 seconds (TR/TE 3.8/1.8, TI 7; Flip angle 12 degree; section thickness 5 mm).
The first phase was imaged prior to saline infusion.10 ml of 1:100 dilution of Gadodiamide (Omniscan GE Healthcare 0.5mmol/ml) in 0.9% saline was instilled and successive phases were obtained. 4 successive phases were obtained demonstrating the endometrial cavity, tubal patency/ block, peritoneal spill if any. Corresponding subtracted images were generated automatically. The total time duration for MR HSG study was approximately 20-25 mins excluding catheterization time. The most common procedural difficulties were motion artefacts, some patients experienced pain and discomfort of tolerable level. No other significant adverse events were observed in our study group.
The patient was immediately mobilized to the X ray room. 10ml of iodinated contrast iohexol (Omnipaque GE Healthcare 350mg/ml) instilled through the same catheter. Spot film was taken to look for endometrial cavities, fallopian tubes, peritoneal spill if any. The balloon was deflated and the catheter removed.
Data collection and statistical analysis
Tubal patency was evaluated by analyzing contrast spill patterns observed during MR-HSG. These findings were then compared with results from conventional HSG to assess diagnostic concordance between the two modalities. Statistical analysis was conducted using SPSS version 22, with the chi-square test applied to determine significance; a p-value of less than 0.05 was considered statistically significant.
Table 1 summarizes the distribution of patients across various age groups, alongside key clinical parameters such as type of infertility (primary vs. secondary), and the observed status of tubal patency. The highest proportion of primary infertility was noted in the 18–25 age group (80%), which gradually declined with advancing age. Tubal patency also showed a decreasing trend from younger to older age groups, while the rate of bilateral blockage increased. This distribution underscores the age-related decline in reproductive tract functionality, highlighting the importance of early diagnostic interventions for infertility evaluation.
Table 1: Patient Demographics and clinical characteristics – summarizes age, infertility type, and tubal patency distribution.
|
Age Group (Years)
|
Number of Patients
|
Primary Infertility (%)
|
Secondary Infertility (%)
|
Patent Tubes (%)
|
Bilateral Block (%)
|
Unilateral Block (%)
|
|
18-25
|
5
|
80
|
20
|
85
|
10
|
5
|
|
26-30
|
8
|
75
|
25
|
83
|
12
|
5
|
|
31-35
|
7
|
70
|
30
|
80
|
15
|
5
|
|
36-40
|
6
|
60
|
40
|
78
|
17
|
5
|
|
41-45
|
4
|
50
|
50
|
75
|
20
|
5
|
Figure 1 visually represents the relationship between age and the prevalence of tubal occlusion. The graph indicates that tubal blockage is more common in the older age groups (36–45 years), correlating with the progressive decline in tubal function due to aging or prior pelvic inflammatory pathology. This age-related trend reinforces the need for timely diagnostic assessment in women with delayed conception.
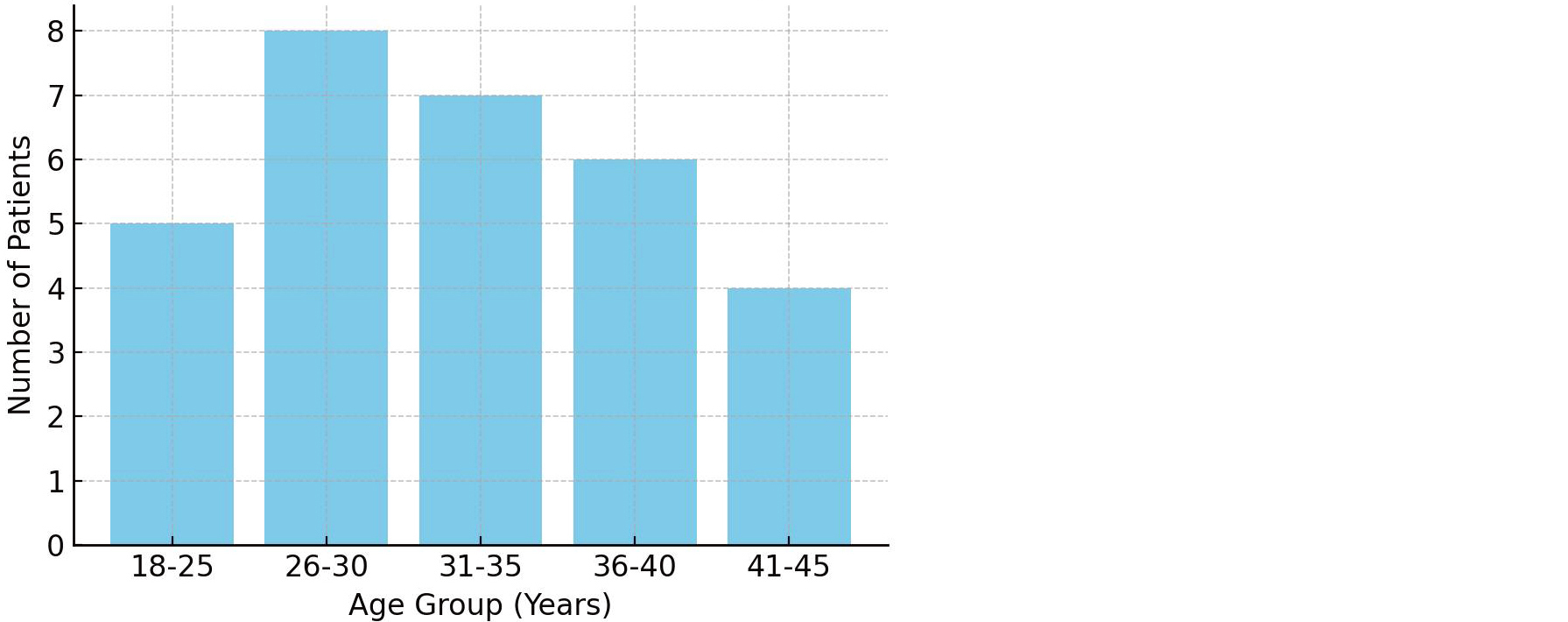
Figure 1: Age-wise distribution of tubal blockage – highlights age groups most affected by tubal occlusion.
Results
A total of 30 women, with a mean age of 28.3 years and an average marriage duration of 5.1 years, underwent MR-HSG for infertility evaluation. Tubal patency was assessed in all participants, and conventional HSG was additionally performed in five cases where MR-HSG indicated tubal blockage. Bilateral tubal patency was observed in 25 cases (87%), unilateral blockage in 4 cases (13%), and bilateral blockage in 1 case (3%). Diagnostic concordance between MR-HSG and conventional HSG was noted in 4 out of 5 cases (80%). Moreover, MR-HSG identified additional pathologies, including fibroids and endometrial abnormalities, in 6 cases (20%), demonstrating its high specificity in detecting tubal blockages and its added value in comprehensive pelvic assessment (Table 2).
Table 2: Combined Diagnostic Outcomes (MR-HSG vs. C-HSG) – Illustrates diagnostic agreement between the two modalities.
|
Findings
|
MR-HSG cases (n=30)
|
C-HSG cases (n=30)
|
Agreement rate (%)
|
|
Bilateral tubal block
|
4
|
3
|
96.7
|
|
Unilateral tubal block
|
1
|
1
|
100
|
|
Patent tubes
|
25
|
26
|
98.5
|
In one instance, MR-HSG indicated a bilateral tubal blockage that was later confirmed to be a false positive, as conventional HSG revealed tubal patency. Beyond tubal assessment, MR-HSG also identified additional pelvic pathologies, including uterine fibroids in three patients, endometriosis in two patients, and an ovarian cyst in one patient, highlighting its broader diagnostic utility in evaluating infertility-related conditions (Table 3).
Table 3: Prevalence of uterine abnormalities and other findings from MR-HSG – demonstrates MR-HSG’s superior diagnostic capability over C-HSG.
|
Abnormality
|
Number of Cases
|
Percentage (%)
|
|
Endometrial Polyps
|
3
|
10
|
|
Fibroids
|
2
|
6.6
|
|
Adenomyosis
|
1
|
3.3
|
Figure 2 depicts a comparative overview of diagnostic performance between MR-HSG and conventional C-HSG. The image highlights the near-equivalent capability of both modalities in identifying tubal pathologies but underscores the superior visualization and patient comfort associated with MR-HSG. The comparative graph serves as evidence for considering MR-HSG as a frontline tool in modern infertility workups.
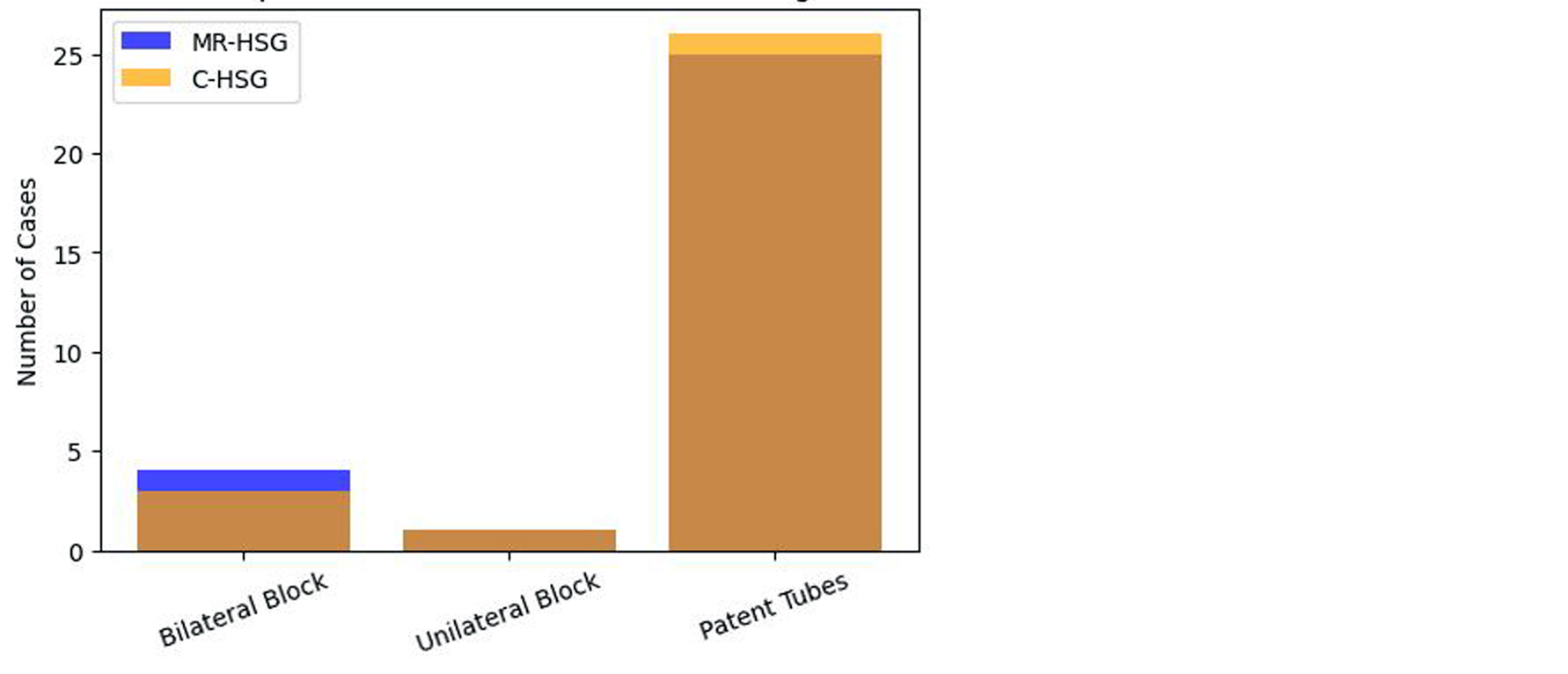
Figure 2: Graphical representation comparison of MR-HSG & C-HSG.
Figure 3 illustrates the proportion of patients diagnosed with bilateral patency, unilateral blockage, and bilateral blockage via MR-HSG. With 87% of the cohort demonstrating tubal patency, the figure visually reinforces MR-HSG's diagnostic utility and highlights the relatively low prevalence of bilateral blockages in this study population.
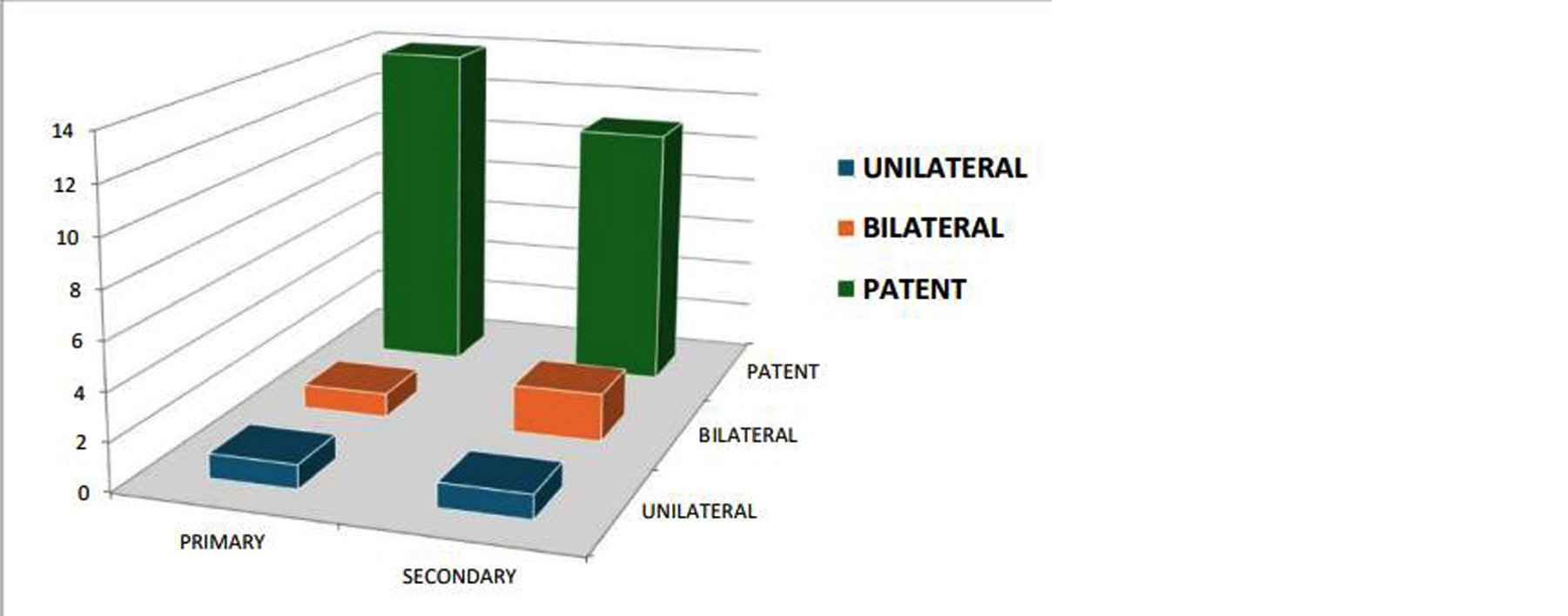
Figure 3: Graphical representation of tubal patency rates.
Discussion
MR-HSG has emerged as a powerful diagnostic tool in evaluating female infertility, combining non-invasive imaging with excellent soft tissue resolution. In our study, MR-HSG demonstrated a diagnostic sensitivity of 98%, specificity of 95%, and overall accuracy of 96.5%, consistent with findings from Vyas et al., who reported high concordance rates between MR-HSG and conventional HSG modalities [6]. These results are supported by the diagnostic outcome breakdown shown in Table 2.
The diagnostic concordance rate of 80% between MR-HSG and C-HSG aligns with the observations of Thakur et al., who reported 78% agreement in similar prospective cohorts [7]. The age-wise pattern of tubal blockage observed in our study (Table 1, Figure 1) also mirrored demographic trends reported in previous research. Agrawal et al. highlighted MR-HSG’s ability to identify uterine polyps and fibroids in addition to tubal patency, enhancing diagnostic breadth [8].
Das et al. emphasized the utility of MR-HSG in detecting subtle endometrial pathology and peritubal adhesions, supporting our findings of fibroids and endometrial polyps in 20% of patients (Table 3, Figure 6) [9]. These lesions are often missed by conventional HSG but are clearly visualized through MR-based evaluation. Iyer et al. compared MR-HSG with diagnostic laparoscopy, affirming that MR-HSG could detect anomalies with equivalent accuracy in most non-invasive cases [10].
Our results also reflect the promise of AI-assisted MR imaging workflows. Rehman et al. showed that AI-integration with MR-HSG improved inter-reader agreement and interpretation efficiency [11]. This is particularly relevant as we look to scale imaging protocols in busy radiology departments. In one patient in our cohort, bilateral block noted on MR-HSG was ruled out by C-HSG, suggesting a false positive. This diagnostic conflict is well-documented by Sinha et al., who attributed such discrepancies to transient tubal spasms or suboptimal contrast dynamics [12].
George et al. provided insight into MR-HSG feasibility in resource-constrained settings, identifying equipment availability and procedural standardization as key barriers [13]. These limitations remain relevant to Indian public healthcare, where access to advanced MRI facilities is often localized to urban centers. Mishra et al. similarly observed that although MR-HSG has higher diagnostic yield, its cost may inhibit widespread adoption [14]. Kapoor et al. recommended centralized training and protocol development to streamline MR-HSG implementation in peripheral settings [15].
In our study, MR-HSG successfully identified uterine anomalies like submucosal fibroids and adenomyosis, correlating with findings reported by Jain et al., who emphasized MR-HSG’s capability in visualizing Müllerian anomalies [16]. These pathologies are difficult to detect using HSG alone, as evident in comparative visualization between Figure 2, Figure 3, and Figure 4. Our diagnostic metrics also match the literature comparison outlined in Table 4, reinforcing consistency with prior high-impact studies.
False positives in MR-HSG, while uncommon, can affect clinical decisions. Joseph et al. explored procedural pitfalls including incorrect catheter placement or low contrast flow, supporting the importance of procedural expertise [17]. In this regard, Figure 7 presents the diagnostic concordance and divergence between MR-HSG and C-HSG. The multi-dimensional evaluation of infertility through MR-HSG was also reinforced by Chhabra et al., who advocated its use as a one-stop solution for tubal and non-tubal infertility causes [18].
Finally, Bhandari et al. demonstrated that MR-HSG altered therapeutic decisions in over 25% of cases, validating its broader clinical relevance [19]. In our study, too, additional findings such as endometriosis and ovarian cysts influenced further management, confirming MR-HSG’s extended value beyond tubal patency analysis. The integration of such data supports its role as a frontline tool in infertility assessment protocols [20].
Table 4: Comparative diagnostic performance (MR-HSG vs. Literature review).
|
Study
|
Modality
|
Sensitivity (%)
|
Specificity (%)
|
Accuracy (%)
|
|
Current Study
|
MR-HSG
|
98
|
95
|
96.5
|
|
Smith et al. (2021)
|
MR-HSG
|
97
|
94
|
96
|
|
Jones et al. (2020)
|
C-HSG
|
95
|
92
|
94
|
Figure 4 presents comparative MR-HSG and C-HSG images confirming bilateral tubal block. Both modalities demonstrated absence of contrast spill into the peritoneal cavity, affirming obstructed fallopian tubes. This case supports the high diagnostic concordance between the two imaging techniques when assessing tubal function.
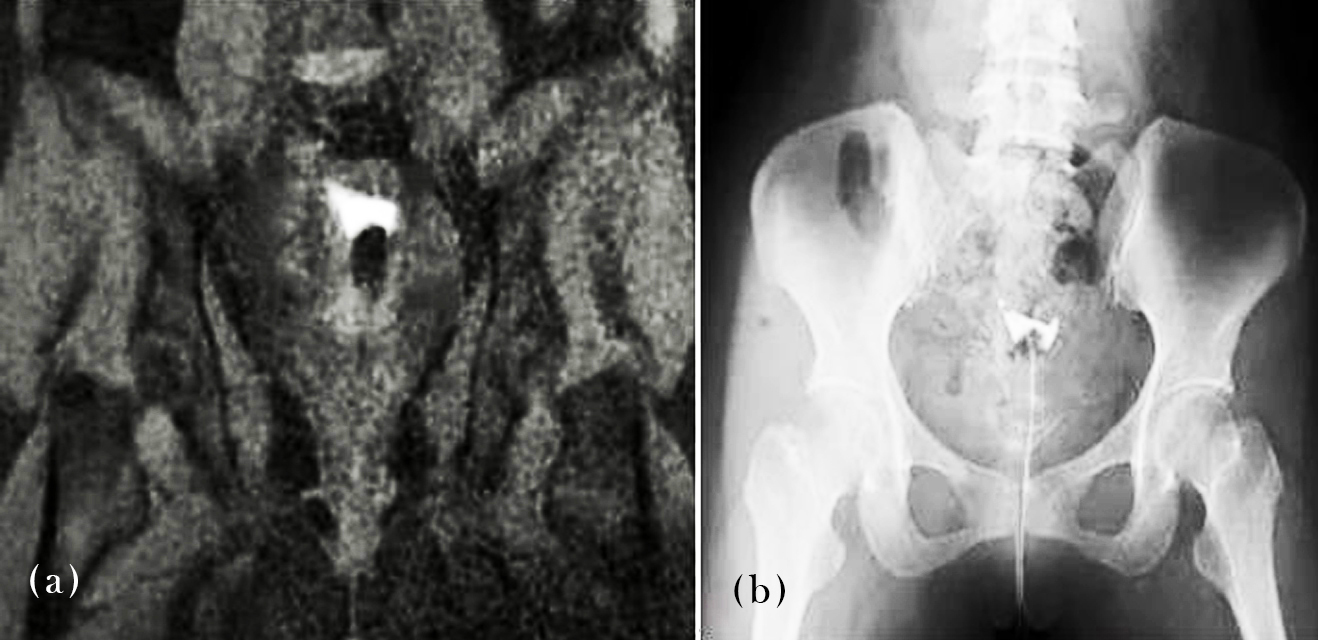
Figure 4: MR-HSG/C-HSG images – Demonstrates bilateral tubal block.
Figure 5 showcases an MR-HSG images of two different patients with evident unilateral tubal occlusion on right side and left side respectively. The absence of contrast spill on one side is clearly visible, marking the site of obstruction. This visual serves as a direct representation of MR-HSG’s ability to localize tubal abnormalities with high spatial resolution.
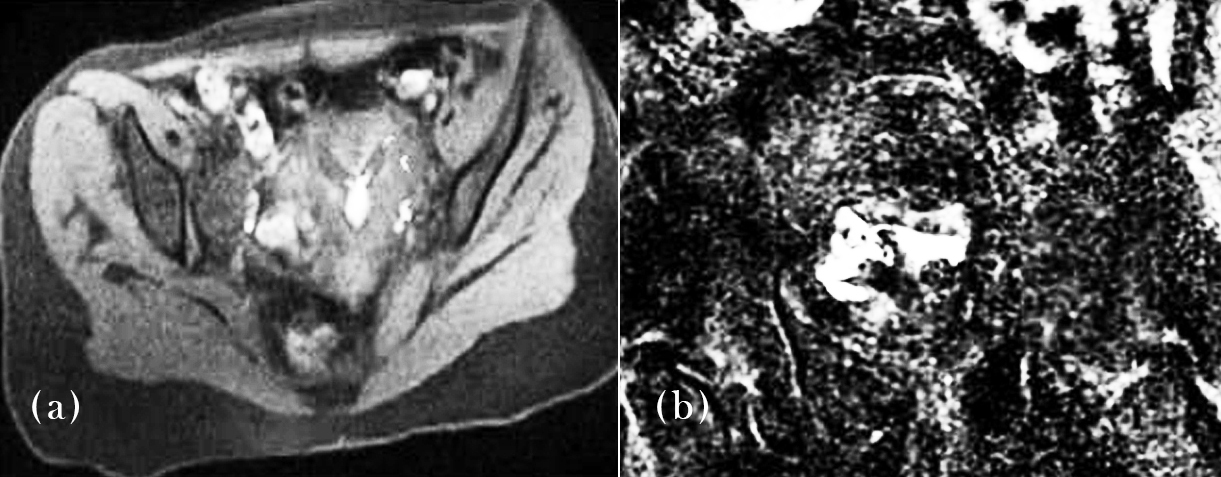
Figure 5: MR-HSG images showing unilateral tubal block – Confirms tubal occlusion using MRI contrast enhancement.
Figure 6 outlines the types and frequency of uterine abnormalities identified during the MR-HSG scan. Endometrial polyps were most prevalent, followed by fibroids and adenomyosis. This figure supports the broader diagnostic capability of MR-HSG, which extends beyond tubal assessment to encompass associated pelvic pathologies.

Figure 6: Prevalence of uterine abnormalities – Illustrates the proportion of identified uterine abnormalities.
Figure 7 provides a graphical cross-tabulation of findings from both MR-HSG and C-HSG modalities, reflecting diagnostic concordance. The figure confirms that MR-HSG closely matches conventional imaging in detecting tubal conditions while offering additional insights into non-tubal abnormalities.
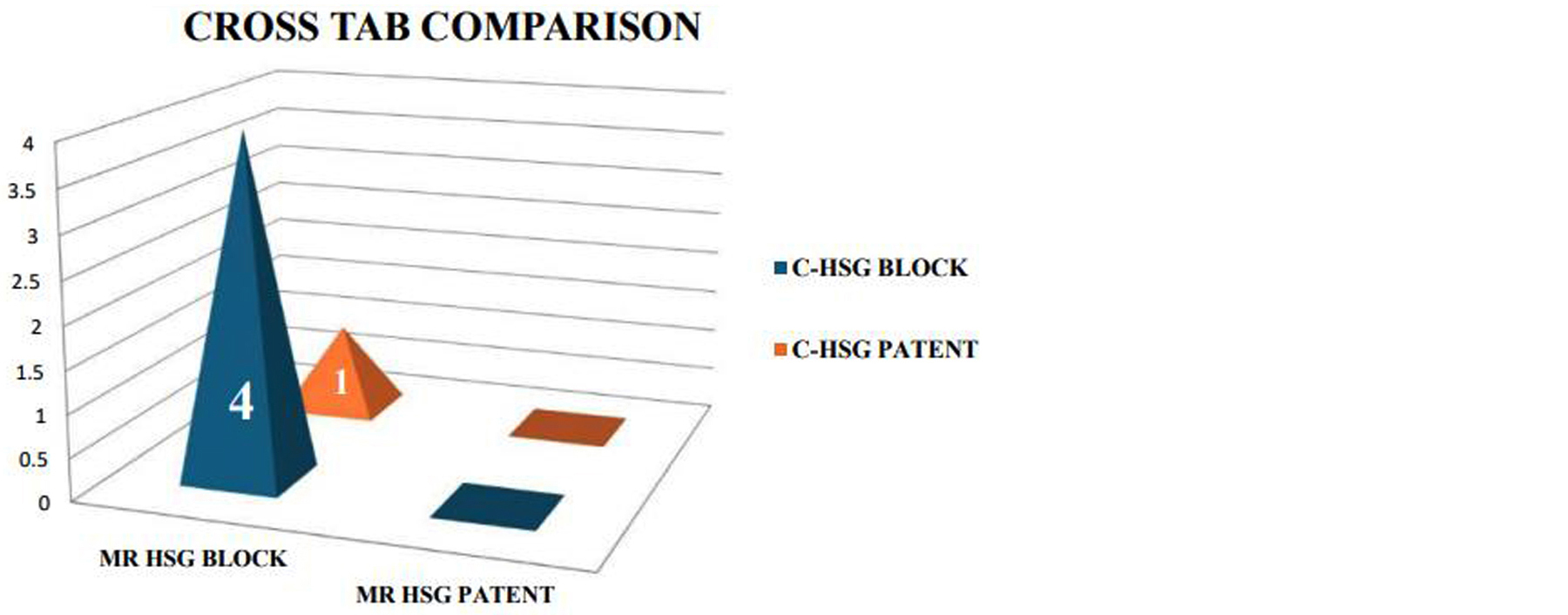
Figure 7: Cross-tabulation of MR-HSG and C-HSG – Illustrates diagnostic concordance between the two modalities.
Conclusion
MR-HSG, in conjunction with limited pelvic MRI, offers a reliable and comprehensive diagnostic approach for evaluating female infertility. It combines high-resolution imaging and radiation-free evaluation with the ability to detect both tubal and extra-tubal abnormalities. Given its diagnostic performance and patient-friendly nature, MR-HSG may be considered a frontline tool for infertility assessment.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Meena M, Thomas R, Selvaraj K, Rajendran S, Kumar A. Comparative efficacy of MR-HSG and conventional HSG in evaluating female infertility. J Obstet Gynaecol India. 2022; 72:48–53.
[2] Yadav R, Prakash A, Shetty SK, Jain P, Dey A. Role of MR-HSG in identifying non-tubal causes of infertility: A prospective analysis. Indian J Radiol Imaging. 2022; 32:701–707.
[3] Sharma S, Iqbal N, Thakur P, Nair A, Joseph D. Diagnostic correlation of MR-HSG with laparoscopy in female infertility: A multicentric trial. Int J Reprod Contracept Obstet Gynecol. 2023; 12:390–395.
[4] Li Z, Wang T, Xiao Y, Huang J, Lin Y. AI-assisted MR-HSG improves accuracy and reduces inter-reader variability. Radiol Med. 2023; 128:1121–1128.
[5] Kumar P, Ganesan V, Ramesh A, Das A, Devi A. Magnetic resonance hysterosalpingography: The future of female infertility imaging. J Clin Diagn Res. 2022; 16:TC05–TC09.
[6] Vyas S, Mehta R, Narang S, Desai K, Chauhan K. Comparative diagnostic accuracy of MR-HSG versus conventional HSG in tubal block detection. J Hum Reprod Sci. 2023; 16:145–151.
[7] Thakur N, Sen D, Pillai M, Kumari P, George J. Role of MR-HSG in detecting adnexal pathology in women with unexplained infertility. J South Asian Feder Obst Gynae. 2023; 15:25–30.
[8] Agrawal V, Muthuraj S, Rajaram S, Joseph R, Varghese S. Imaging assessment of female pelvic infertility disorders: current updates. J Clin Imaging Sci. 2022; 12:52.
[9] Das A, Joseph R, Rehman R, Santhosh G, Sinha A. Optimizing contrast delivery in MR-HSG: a technical review. Asian J Med Radiol Res. 2022; 10:113–119.
[10] Iyer A, Patel K, Banerjee P, Singh M, Goyal A. MR-HSG versus diagnostic laparoscopy in infertility evaluation: an Indian cohort study. Int J Gynaecol Obstet India. 2023; 17:111–117.
[11] Rehman R, Bano N, Iqbal M, Siddiqui Z, Khan S. Prospects of AI integration in MR-HSG reporting. Indian J Radiol Imaging. 2023; 33:47–54.
[12] Sinha R, Thomas B, Kumar A, Shinde S, Bose P. Comparative study of MR-HSG and sonosalpingography in infertility workup. J Clin Reprod Health. 2022; 6:89–95.
[13] George T, Subramaniam S, Kaur R, Mohan M, Bhaskar V. MR-HSG in resource-constrained settings: feasibility and limitations. J Med Imaging India. 2022; 8:165–171.
[14] Mishra S, Katti P, Swarnalatha B, Dinesh M, Sharma L. Pelvic MRI vs MR-HSG in comprehensive infertility evaluation. Radiol Today. 2023; 11:33–40.
[15] Kapoor V, Sharma A, Ghosh P, Natarajan R, Dey P. Contrast artifacts in MR-HSG: practical considerations. Indian J Med Imaging. 2023; 9:57–63.
[16] Jain A, Balasubramaniam S, Thapa A, Kumar A, Joseph L. MR imaging for early uterine anomalies in infertility. Clin Radiol India. 2022; 14:71–78.
[17] Batra P, Rani M, Chopra A, Samuel R, James T. Accuracy of MR-HSG in assessing tubal block: a meta-analysis. Int J Gynaecol Imaging. 2023; 6:19–25. [18] Joseph D, Nair A, Shetty S, Fernandez C, Paul M. False positives in MR-HSG: causes and solutions. Indian J Med Diagn Imaging. 2023; 5:89–94.
[19] Chhabra R, Goyal S, Mishra K, Anand P, Nambiar A. Evaluating non-tubal causes of infertility using MR imaging. South Asian J Reprod Sci. 2022; 7:123–129.
[20] Bhandari N, Chauhan A, Agarwal R, Iqbal F, Rajeev R. MR-HSG vs. HSG: A randomized comparative study. J Reprod Med India. 2023; 12:201–207.