Abstract
Background: Breast cancer, the second most common worldwide, causes significant morbidity and mortality in developing nations. Aberrant HSP-27 expression links to aggressive tumors, chemotherapy resistance, and poor prognosis. The stage of disease at diagnosis is the most important prognostic variable. Hence, this study was undertaken to evaluate the expression of HSP-27 in Invasive breast carcinoma and metastatic regional lymph nodes.
Methods: The present study was a prospective study conducted from December 2019 to May 2021. Data was obtained from clinical records of 35 patient’s preliminary diagnosed as breast carcinoma by different diagnostic modalities Corresponding modified radical mastectomy specimens were grossed according to standard protocol ,processed to paraffin block and sectioned. The slides were stained with routine Haematoxylin and eosin stain. Additionally, the slides were immunostained with HSP-27 antibody.
Results: The study included 35 female patients aged 29 to 80 years, with a mean age of 50 years. Most cases (37%) were between 31–40 years. Invasive ductal carcinoma, no special type, was the most common histopathologic pattern (97%). Grade II tumors (46%) and TNM stage IIIA (34%) predominated. Positive HSP-27 expression was noted in 89% of breast carcinomas and 100% of metastatic lymph nodes. Expression intensity varied, but no significant correlation was found with tumor grade or stage (p > 0.05).
Conclusion: HSP-27 can be a reliable tumor marker in breast carcinoma and may be a molecular target in cancer therapy.
Keywords: breast carcinoma; metastatic lymph node; heat shock protein-27
Full Text
Introduction
Breast cancer is the most common malignancy in women and is second only to lung cancer as a cause of cancer deaths worldwide [1]. Every year 75,000 new cases of breast cancer are diagnosed in Indian women [2]. The average age at diagnosis is lower in developing countries than in European and American populations [3].
Breast carcinomas are a heterogeneous group of tumors and the earlier classification of breast carcinomas was based on clinical and pathological characteristics. Understanding cancer at the molecular level has led to molecular classification with discovery of new subclasses of tumors not appreciable by morphology [3].
As Molecular approaches to breast carcinoma have assumed greater prognostic and predictive importance it will be important to evaluate specific therapeutic molecular targets in tumours to determine the appropriateness of a particular therapy for a given patient [3]. One of the emerging novel molecular targets is Heat Shock Protein-27 due to its diverse function in cells. HSP-27 is a member of HSP family which is involved in cell migration, cell growth/ differentiation, tumor progression and also has anti-apoptotic property Its aberrant expression in breast cancer is associated with aggressive tumor behavior, increased resistance to chemotherapy and poor prognosis for the patients [4].
There is paucity of literature regarding expression of HSP-27 in breast carcinoma in India. Hence, this study was undertaken to evaluate the expression of HSP-27 in breast carcinoma and in metastatic regional lymph nodes.
Methods
A cross-sectional study was done in the tertiary care centre after from December 2019 to May 2021 on the modified radical mastectomy specimens of patients with a preliminary diagnoses of breast carcinoma by various diagnostic modalities after obtaining the ethics committee approval. In the present study, a total of 35 cases of breast carcinomas with metastatic axillary lymph nodes were included in the study as the estimated sample size was 30 based on prevalence of 0.92, α error of 5% and absolute error of 10%. The clinical diagnosis, demographics (age, sex) and clinical features (laterality, tumour topography) were noted from the clinical records.
Cases of breast carcinoma with positive lymph node were included and cases in which lymph nodes were negative for metastasis and tumours with extensive necrosis were excluded. The standard protocol for surgical grossing of modified radical mastectomy specimen was followed. After conventional processing, paraffin sections of 5µm thickness were stained by haematoxylin and eosin for histopathological examination.
In addition, sections of 4µm thickness were made and taken on poly-L-Lysine (PLL) coated slides. Immunohistochemistry for HSP-27 was performed on tissue sections using HSP-27 monoclonal antibody. Heat antigen retrieval was done by using TRIS EDTA buffer by pressure cooker method for HSP-27 monoclonal antibody and standard immunohistochemistry procedure was performed according to the manufacturer’s instructions.
Histological types were determined and categorized according to the WHO 2012 classification. Grading of the tumors was done by Elston and Ellis’ modification of Scarff Bloom Richardson grading system and Staging was done according to TNM staging. For evaluation of HSP-27 expression, the scoring system applied was immunoreactive scale of Remmele [5] (Table 1) and the final score was obtained by multiplying Score A and Score B. Score of +1 or more was considered as positive reaction.
Table 1: Remmele scale: percentage of positive cells (A) and the intensity of colour reaction (B). The final score represents the product of these parameters (A×B) AB.
|
A
|
B
|
|
0 pts — no cells with positive reaction
|
0 pts — no staining
|
|
1 pt — to 10% cells with positive reaction
|
1 pt — low intensity of staining
|
|
2 pts — 11–50% cells with positive reaction
|
2 pts — moderate intensity of staining
|
|
3 pts — 51–80% cells with positive reaction
|
3 pts — intense staining
|
|
4 pts — > 80% cells with positive reaction
|
|
The data collected was statistically evaluated and represented in terms of frequency distribution tables. The categorical and counting variables were presented by frequencies and percentages. The proportion of subjects revealing the expression according to the various subgroups such as grade and stage was estimated. Correlation of HSP-27 expression was assessed with the grade and stage of the breast carcinoma.HSP-27 expression between Primary tumour and axillary lymph nodes was compared.
The differences in frequency of expression between various subgroups were tested for statistical significance by employing chi Square/Fischer test. P ≤ 0.05 was considered statistically significant.
Results
The present study included a total of 35 cases of breast carcinoma metastatic to the regional lymph nodes diagnosed during the study period.
The age incidence of the patients with breast carcinoma ranged in age from 29 years to 80 years, the most common age group was 31 to 40 years (37 %) followed by 41 to 50 years (26%) and was significantly rare after 70 years. In this study, on gross examination, the size of the tumors varied from 1.8 cm to 11.5 cm. Majority of the tumors 27 (77%) measured between 2 to 5 cm followed by tumors 6 (17%) with size more than 5 cms and tumors 2 (6%) less than 2cms. Number of Axillary lymph nodes positive for malignancy varied from 1 to 22 lymph nodes. All the cases 35(100%) were diagnosed as Invasive ductal carcinoma of no special type (IDC NST) on histopathology (Table 2).
Table 2: Clinicopathological characteristics of breast carcinoma cases.
|
Variables
|
|
Number of cases (n)
|
Percentage (%)
|
|
Gender
|
Female
|
35
|
100%
|
|
Age range
|
<40 years
|
13
|
37%
|
|
41-50 years
|
09
|
26%
|
|
51-60 years
|
05
|
14%
|
|
61-70 years
|
06
|
17%
|
|
71-80 years
|
01
|
3%
|
|
>80 years
|
01
|
3%
|
|
Laterality
|
Right sided
|
14
|
40%
|
|
Left sided
|
21
|
60%
|
|
Histological type
|
IDC-NST
|
35
|
100%
|
|
Number of lymph nodes involved
|
1-3
|
13
|
37%
|
|
4-9
|
12
|
34%
|
|
>10
|
10
|
29%
|
|
Size of the tumour
|
≤ 2 cm
|
2
|
6%
|
|
2-5 cm
|
27
|
77%
|
|
> 5 cm
|
6
|
17%
|
Modified SBR grade‑II was observed in16 (46 %) of the total 35 MRM specimens followed by grade I and grade III seen in 15 (43 %) and 4 (11%) respectively. With TNM staging it was observed that majority of the cases belonged to pathological stage III 24 (69%) followed by stage II 11(31%) (Table 3).
Table 3: Grade wise and stage wise distribution of breast carcinoma.
|
Grade
|
|
Number of cases
|
Percentage
|
|
Grade 1/Well differentiated
|
15
|
43%
|
|
Grade 2/Moderately differentiated
|
16
|
46%
|
|
Grade 3/Poorly differentiated
|
04
|
11%
|
|
Staging
|
I
|
-
|
-
|
|
IIA
|
-
|
-
|
|
IIB
|
11
|
31%
|
|
IIIA
|
12
|
34%
|
|
IIIB
|
2
|
6%
|
|
IIIC
|
10
|
10%
|
|
IV
|
-
|
-
|
Distribution of breast carcinoma as per Immunohistochemistry findings (Table 4): Majority of IDC (NST) 16 (46%) had a score of 3+, followed by score of 2+in 8 (23%) and 1+ in 7 (20%).
Table 4: HSP-27 expression in relation to breast carcinoma.
|
Histological diagnosis
|
Score 0/ negative
|
Score 1+
|
Score 2+
|
Score 3+
|
Total positive cases
|
|
Invasive carcinoma-NST
|
4
|
7
|
8
|
16
|
31/35
|
Immunohistochemistry findings with regard to grading of tumours (Table 5): Of 16 MRM specimens of IDC (NST) grade‑II 8(50 %) had a score of 3+ followed by score of 2+ in 4(25%) and 1+ in 2(6%). 2(12%) were negative for HSP-27 expression.
Table 5: HSP-27 expression in relation to grade of breast carcinoma.
|
Grade
|
Score 0/negative
|
Score 1+
|
Score 2+
|
Score 3+
|
Total no. of cases
|
Percentage
|
|
Grade 1/ Well differentiated carcinoma
|
02
|
05
|
04
|
04
|
15
|
43%
|
|
Grade 2/ Moderately differentiated carcinoma
|
02
|
02
|
04
|
08
|
16
|
46%
|
|
Grade 3/ Poorly differentiated carcinoma
|
-
|
-
|
-
|
04
|
04
|
11%
|
Of 15 MRM specimens of IDC (NST) grade‑I, 4(26%) had a score of 3+ and 4(26%) had a score of 2+ followed by a score of 1+ in 5(13 %).13% were negative for HSP-27 expression. In 4 MRM specimens of IDC NST grade‑III a score 3+ was observed. Correlation between grade of the carcinoma and HSP27 expression was found not to be significant (P value > 0.05).
Immunohistochemistry findings with regard to staging of tumours (Table 6): IDC NST stage‑III A had a score 3+ in 6(50 %) followed by score of 2+in 3 (25 %) and 1+ in 1 (8%) of the total 12 MRM specimens.2 (16%) were negative for HSP expression A score of 3+ was observed in5 (47 %) followed by score of 2+ in 2(18 %) and 1+ 3 (27%) of the total 11 MRM specimens with IDC NST stage‑IIB.10 % were negative for HSP expression A score 3+, 2+ and 1+ was observed in 3 (30 %) each of the total 10 MRM specimens with IDC NST Stage ‑IIIC. 1(9%) was negative. In IDC NST stage‑III B a score 3+ was seen in 2 MRM specimens. Correlation between stage of the carcinoma and HSP27 expression was found not to be significant (P value > 0.05).
Table 6: HSP-27 expression in relation to stage of breast carcinoma.
|
Stage
|
Score 0/negative
|
Score 1+
|
Score 2+
|
Score 3+
|
Total no. of cases
|
|
I
|
-
|
-
|
-
|
-
|
0
|
|
IIA
|
-
|
-
|
-
|
-
|
0
|
|
IIB
|
1
|
3
|
2
|
5
|
11
|
|
IIIA
|
2
|
1
|
3
|
6
|
12
|
|
IIIB
|
-
|
-
|
--
|
2
|
02
|
|
IIIC
|
1
|
3
|
3
|
3
|
10
|
|
IV
|
-
|
-
|
-
|
-
|
0
|
HSP-27 expression in metastatic axillary lymph nodes (Table 7): Metastatic lymph nodes of all 35 cases of breast carcinoma displayed HSP-27 expression including the 4 cases with negative HSP-27 expression in primary breast carcinoma.
Table 7: HSP-27 expression in node- positive breast carcinoma.
|
HSP-27 expression
|
Number of cases
|
Percentage
|
|
HSP-27 expression in breast carcinomas
|
Positive
|
31
|
89%
|
|
Negative
|
04
|
11%
|
|
HSP-27 expression in lymph nodes
|
Positive
|
35
|
100%
|
|
Negative
|
0
|
-
|
A high-power microscopic examination demonstrates a mild (1+) level of HSP-27 expression in a case of invasive breast carcinoma of no special type (NST), indicating weak immunoreactivity within the tumor cells. (Figure 1). Microscopic examination at high magnification reveals a moderate (2+) intensity of HSP-27 immunohistochemical staining in tumor cells of well-differentiated invasive breast carcinoma, accompanied by the presence of characteristic comedo necrosis within the lesion (Figure 2). Microscopic view reveals strong (3+) HSP-27 expression in moderately differentiated invasive breast carcinoma, no special type (NST) (Figure 3). Microscopic examination of the lymph node reveals metastatic tumor cell deposits located in the subcapsular region, exhibiting positive immunohistochemical staining for HSP-27 expression, confirming the presence of metastatic involvement (Figure 4). Histopathological examination of the lymph node demonstrates metastatic tumor deposits with evident positive immunoreactivity for HSP-27, indicating the spread of carcinoma cells to the nodal tissue (Figure 5).
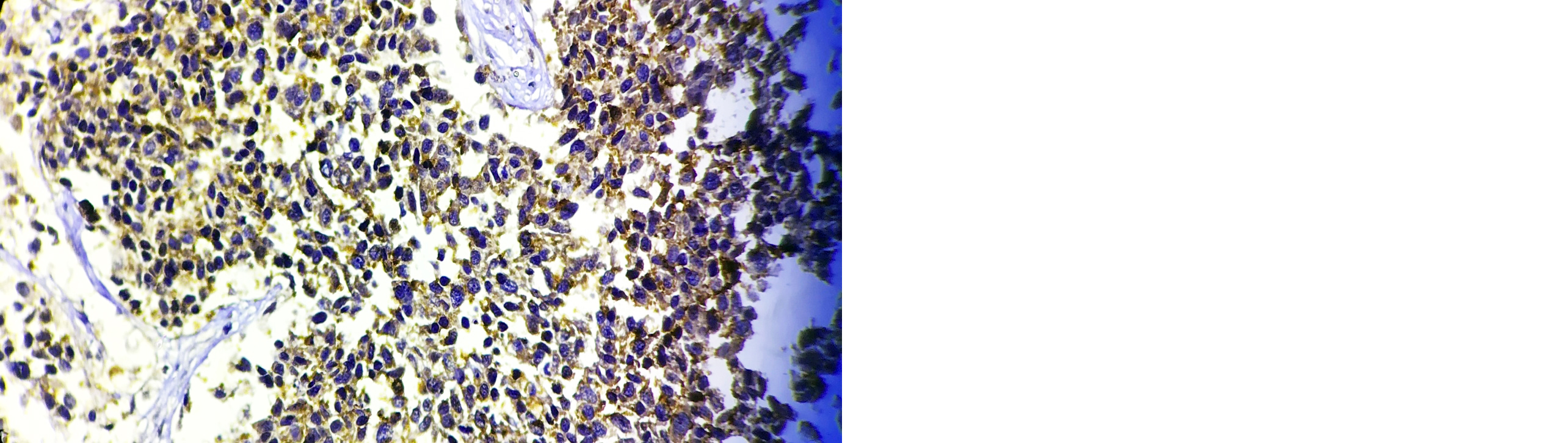
Figure 1: Microscopic image of HSP-27 expression showing 1+ score in Invasive breast carcinoma-NST (40X).
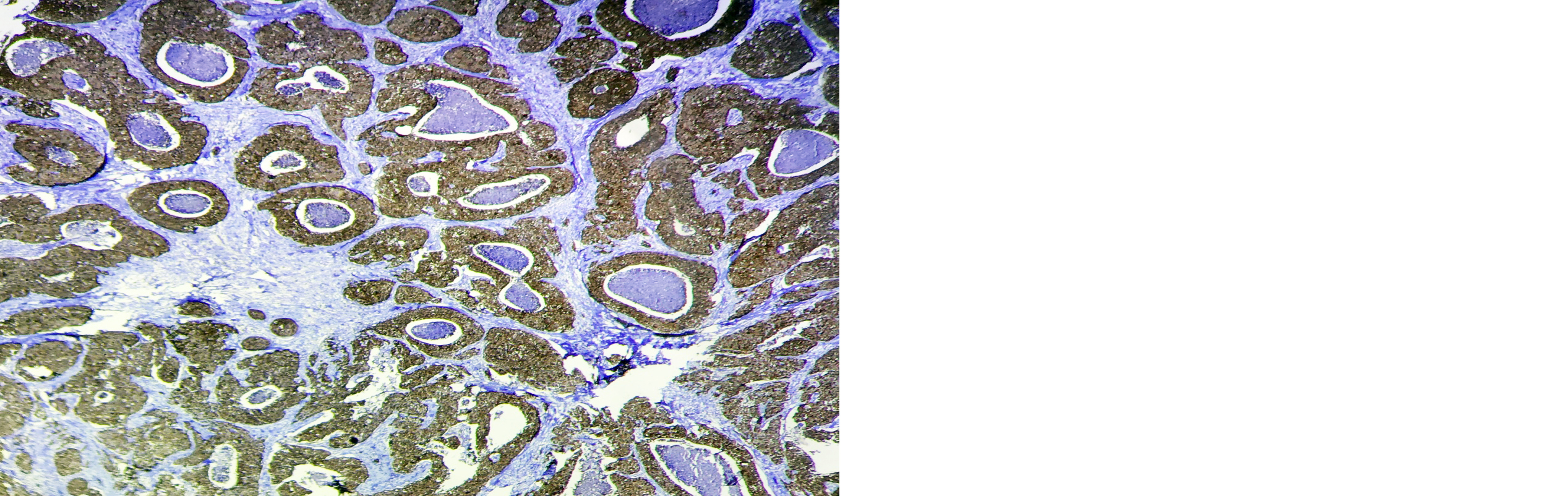
Figure 2: Microscopic image of HSP-27 expression showing 2+ score in well-differentiated Invasive breast carcinoma with comedo necrosis (4X).
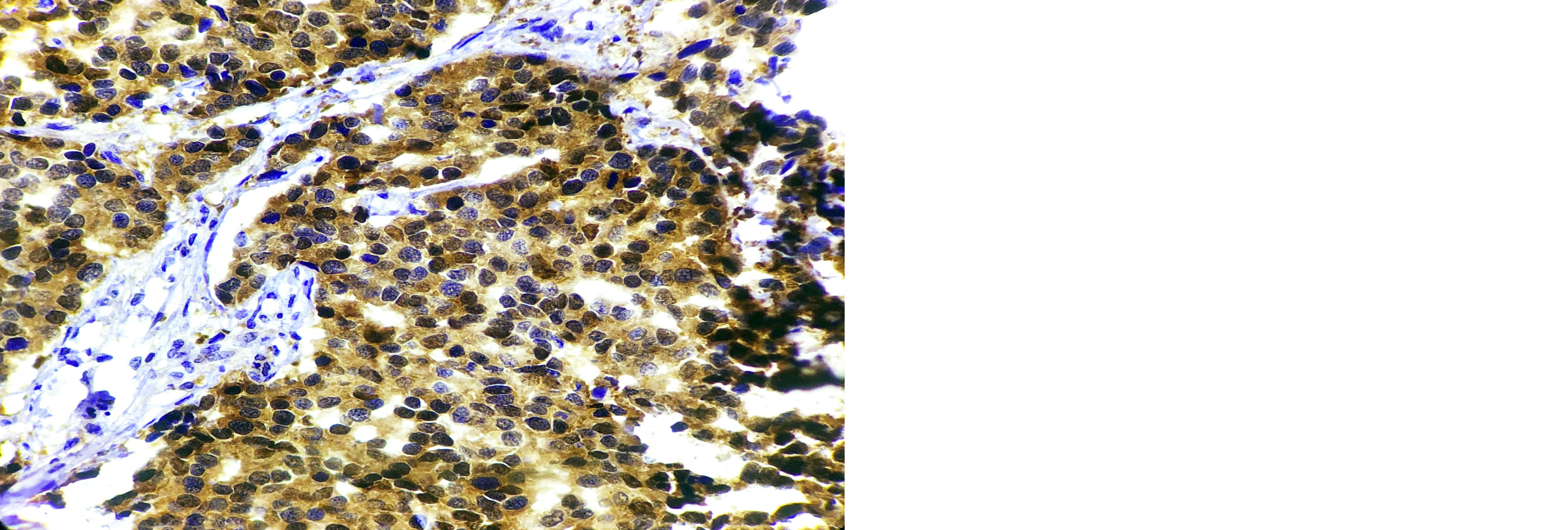
Figure 3: Microscopic image of HSP-27 expression showing 3+ score in Moderately differentiated Invasive Breast Carcinoma-NST ( 40X).
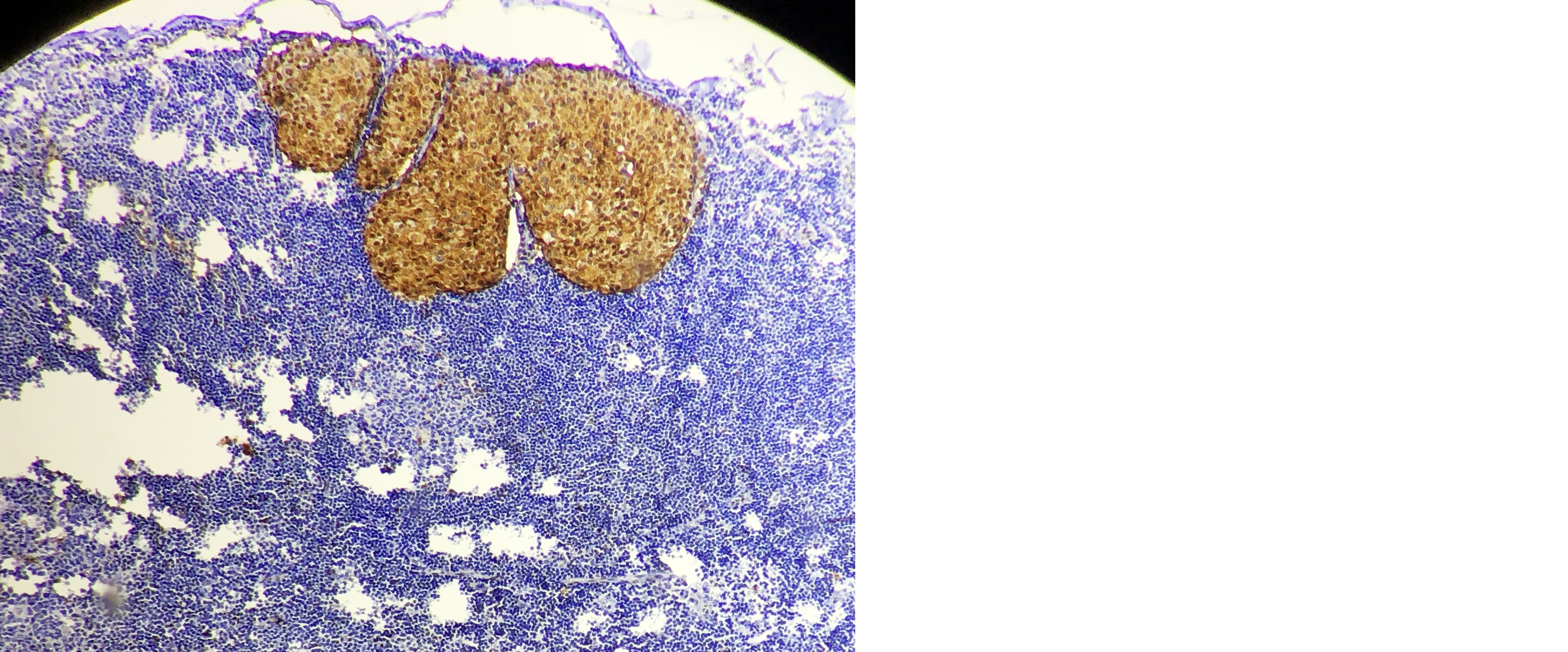
Figure 4: Microcopic picture of lymph node showing subcapsular metastatic deposits positive for HSP-27 expression (10X).
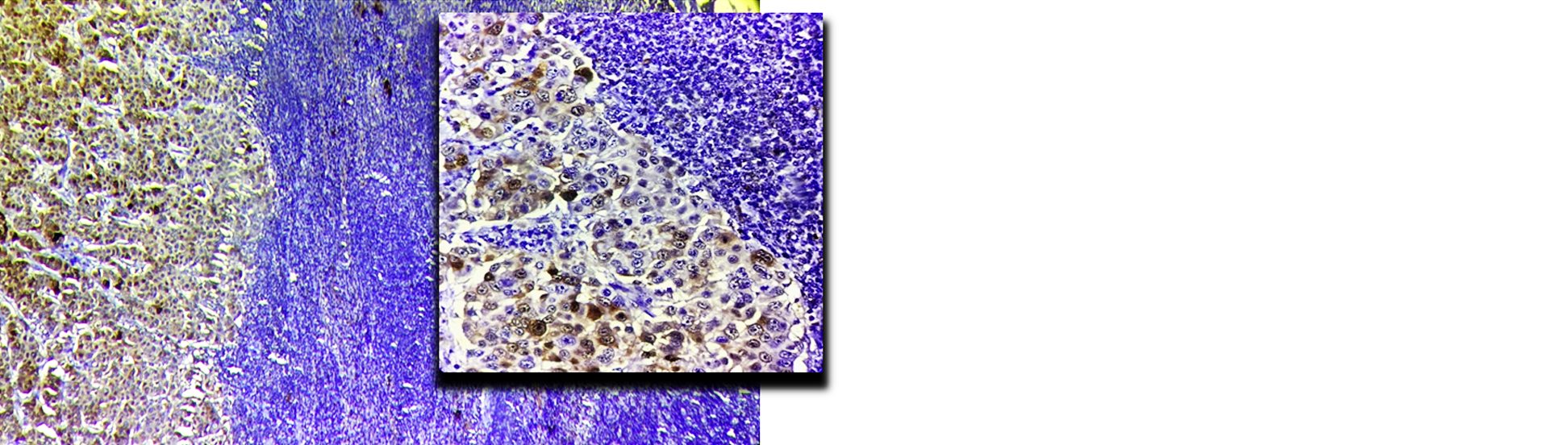
Figure 5: Microscopic image of lymph node showing metastatic deposits positive for HSP-27 expression (10X, Inset 40 X).
Discussion
Epidemiology of breast cancer across India is showing increasing trends for incidence and mortality mainly due to rapid urbanization, industrialization, population growth and ageing. Also, delayed disease presentation due to illiteracy, lack of awareness, financial constraints, lack of organized breast cancer screening program and paucity of diagnostic aids put extra load on the existing infrastructure.
There are subsets of breast carcinoma which are not susceptible to conventional therapy and have a paradigm shift in molecular genetics and immunohistochemical expression. These are the Basal-like breast carcinomas and the triple negative breast cancers.
Heat shock protein 27 is a molecular chaperone with cytoprotective and anti-apoptotic capacities [7, 8]. Its overexpression in malignant tissues and various tumor cell lines is associated with altered cell growth and elevated tumorigenicity [9, 10]. It is correlated with decreased survival in ovarian cancer, but also with favorable outcome in patients with malignant fibrous histiocytoma or endometrial cancer [11, 12]. But, there has been contradictory results on the studies done on prognostic and predictive value of HSP-27 in breast carcinomas (Table 8). In the present study, cases were distributed between the age group of 29 to 90 years.
Table 8: Comparing the mean age at presentation with other studies.
|
Authors
|
Mean age (years)
|
|
Zeinalian et al [13]
|
44.2
|
|
Malik et al [14]
|
47.7
|
|
Present study
|
50
|
Majority of cases belonged to the age group of <40 years with mean age of 50 years. The occurrence of breast carcinoma above 50 years of age in our study constituted 23% cases, which is different from western literature that depicts nearly 75.0% of patients with breast cancer are above 50 years of age [15]. The mean age at diagnosis in our study was higher when compared with other studies. According to our study 60% of the cases were left sided and the rest were right sided (Table 9).
Table 9: Comparing of Laterality of breast carcinoma with other studies.
|
Authors
|
Left side
|
Right side
|
Bilateral
|
|
Zeinalian et al [13]
|
50.4%
|
46.1%
|
3.5%
|
|
Badru et al [16]
|
52%
|
48%
|
-
|
|
Present study
|
60%
|
40%
|
-
|
Breast carcinoma was found to be more common in the left breast consistent with few other studies. The left breast is somewhat larger than the right, and this reason was used by other studies to explain the higher incidence of breast carcinoma on left side [13, 14].
Tumour size is an important prognostic factor and indicator for overall survival as clearly shown by its inclusion in the TNM Staging System. Of these 35 cases, majority (67%) subjects have tumour size between 2 and 5 cm which agrees with the study conducted by Mohapatra et al and Pradhan et al [17, 18]. In a study by Ahmad et al [9], majority of the cases were > 5cms which could be result of delayed medical attention (Table 10).
Table 10: Comparing tumour size with other studies.
|
Authors
|
<2cms
|
2-5cms
|
>5cms
|
|
Mohapatra et al [17]
|
5%
|
75.6%
|
19.4%
|
|
Pradhan et al [18]
|
9.7%
|
54.8%
|
35.5%
|
|
Ahmad et al [19]
|
8%
|
44%
|
48%
|
|
Present study
|
6%
|
77%
|
17%
|
The most frequently observed histopathological pattern was invasive carcinoma NST, 35 cases (100%) (Table 11).
Table 11: Comparing HSP-27 expression in breast carcinoma with other studies.
|
Study
|
Total number of cases
|
HSP-27 positive
|
HSP-27 negative
|
Percentage of positive cases
|
|
Grzegrzolka et al [20]
|
101
|
92
|
09
|
92%
|
|
Thanner et al [21]
|
191
|
157
|
34
|
82%
|
|
Tetu et al [22]
|
890
|
383
|
507
|
43%
|
|
Storm et al [23]
|
30
|
09
|
21
|
30%
|
|
Present study
|
35
|
31
|
04
|
89%
|
In the present study, HSP-27 expression does not correlate with histopathological grade of the carcinoma (p value= 0.239). Although, the study conducted by J Grzegrzolka et al [20]showed HSP-27 expression was almost significantly higher in G3 than in G1 cases and thus established statistically significant correlation with histopathological grading (p value=0.0524) which conflicts the data published by Love et al [81] (Table 12).
Table 12: Comparing the correlation of HSP-27 expression with histopathological grade of breast carcinoma with other studies.
|
Study
|
Total number of cases
|
Grade 1
|
Grade 2
|
Grade 3
|
P value
|
|
Grzegrzolka et al [20]
|
101
|
09
|
52
|
40
|
0.0524
|
|
Thanner et al [21]
|
191
|
128
|
50
|
0.077
|
|
Love et al [24]
|
361
|
-
|
0.04
|
|
Present study
|
35
|
15
|
16
|
04
|
0.239
|
In astrocytoma and hepatocellular cancer, HSP-27 expression was positively correlated with grade of malignancy [25, 26]. However, no significant correlation between grade of malignancy and level of HSP-27 expression in ovarian cancer, squamous cell carcinoma of the tongue and esophagus, bladder carcinoma or gastric cancer was observed [27-29]suggesting that the expression of HSP-27 has a heterogeneous character and is specific for the type of tumour.
HSP-27, which is also known as a cytoplasmic estrogen receptor associated protein (p29), selectively binds GTP and to a lesser extent ATP and plays a role in the estrogen response intracellular pathways [30].
Out of 35 cases of breast carcinoma with regional metastatic lymph nodes, 4 cases with no or low HSP-27 expression in primary breast carcinoma displayed high HSP-27 expression in the metastatic axillary lymph nodes. Hence, the concordance level between the tumor mass and the lymph node was 88.57%.
Similar results were seen in a study by Storm et al [23]. If we accept that HSP-27 overexpression is cytoprotective, all cells that shed from the primary cancer to the regional nodes, those cells expressing HSP-27 have a survival advantage.
An alternative hypothesis is that lymph nodes are a uniquely hostile environment that stimulates cancer cells to overexpress this stress-response protein. In either case, whether by a process of selection or induction- facilitation, HSP-27 overexpression appears to confer protection for metastatic cells, which may help explain why HSP-27 overexpression is associated with reduced disease-free survival in breast carcinomas [23].
Several studies have demonstrated that the overexpression of HSP-27 seems to be correlated with increased resistance to chemotherapeutic drug-induced apoptosis in cancer cells. Hansen et al [31]. reported the inhibition of doxorubicin induced apoptosis in the HSP-27 overexpressing breast cancer cell, demonstrating a protective role of HSP-27 against apoptosis. Also, upregulation of HSP-27 in breast cancer cells reduces trastuzumab susceptibility by increasing HER2 protein stability [32, 33]. These recent studies suggest possibility of HSP-27 inhibition as molecular target for cancer therapy [6].
Conclusion
In the current study, HSP-27 expression was found in the majority of patients with breast carcinoma 31 (89%) and synchronous metastatic axillary lymph nodes 35 (100%) with a concordance level of 88.57% between the tumor mass and the lymph node. Nevertheless, there was no statistically significant correlation (p > 0.05) between the grade and stage of breast carcinoma and the expression of HSP 27. Therefore, HSP-27 can be used as a reliable tumour marker in breast carcinoma and probably as molecular target in cancer therapy. With these promising results, further studies are warranted with larger sample size and follow-up period.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Chapter 23, The Breast. Philadelphia: Elsevier; 2022. p.1051.
[2] Ferlay J, Soerjomataram I, Dikshit R, Ese S, Maters C, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
[3] Giordano TJ, Schottenfeld D, Fraumeni JF. Morphologic and molecular classification of human cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. p.11.
[4] Lee S‑u Kim, Jun Ho Kim. Heat shock proteins as molecular targets for breast cancer therapeutics. J Breast Cancer. 2011; 14:167–174.
[5] Remmele W, Stegner HE, et al. Recommendation for uniform definition of an immunoreactive Score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer. Pathologe. 1987;8:138–140.
[6] Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA, et al. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993; 85:1558–1570.
[7] Benndorf R, Bielka H. Cellular stress response: stress proteins – physiology and implications for cancer. Rec Res Cancer Res. 1997; 143:129–144.
[8] Ciocca DR, Adams DJ, Edwards DP, Bjercke RJ, McGuire WL, et al. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983; 43:1204–1210.
[9] Morino M, Tsuzuki T, Ishikawa Y, Shirakami T, Yoshimura M, et al. Specific expression of hsp27 in human tumor cell lines in vitro. In Vivo. 1997; 11:179–184.
[10] Langdon SP, Rabiasz GJ, Hirst GL, King JB, Hawkins RA, et al. Expression of the heat shock protein hsp27 in human ovarian cancer. Clin Cancer Res. 1995; 1:1603–1609.
[11] Geisler JP, Geisler HE, Tammela J, Miller GA, Wiemann MC, et al. A study of heat shock protein 27 in endometrial carcinoma. Gynecol Oncol. 1999; 72:347–350.
[12] Zeinalian M, Heidarzadeh N, Naji H, Sharbafchi MR. Clinicopathological analysis of patients with breast cancer and their families. Iran J Blood Cancer. 2016; 8:17–22.
[13] Malik IA. Clinico‑pathological features of breast cancer in Pakistan. J Pak Med Assoc. 2002; 52:100–104.
[14] Lester SC, Kumar V, Abbas A, Fausto N. The Breast. In: Kumar V, Abbas A, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed. India: Saunders; 2004. p.1131–1146.
[15] Badru F, Chianakwalam C, Stevenson V. Laterality of breast cancer – is it true? Eur J Surg Oncol. 2011; 37:987.
[16] Mohapatra M, Satyanarayana S. Evaluation of clinicopathologic findings of breast carcinoma in a general hospital in Southern India. Indian J Cancer. 2013; 50:297.
[17] Pradhan A, Paudyal P, Sinha AK, Agrawal CS. Grading, staging and Nottingham prognostic index scoring of breast carcinoma. J Pathol Nepal. 2017; 7:1078–1083.
[18] Ahmad Z, Khursid A, Qureshi A, Idress R, Asghar N, et al. Breast carcinoma grading, estimation of tumor size, axillary lymph node status, staging and Nottingham prognostic index scoring on mastectomy specimens. Indian J Pathol Microbiol. 2009; 52:477–481.
[19] Grzegrzolka J, Kurnol K, Piotrow P, Pula B, Kobierzycki C, et al. HSP27 expression in invasive ductal breast carcinoma. Folia Histochem Cytobiol. 2012; 50:527–533.
[20] Thanner F, Sutterlin MW, Kapp M, Rieger L, Morr AK, et al. Heat shock protein 27 is associated with decreased survival in node‑negative breast cancer patients. Anticancer Res. 2005; 25:1649–1654.
[21] Tetu B, Brisson J, Landry J, Huot J. Prognostic significance of heat‑shock protein‑27 in node‑positive breast carcinoma: an immunohistochemical study. Breast Cancer Res Treat. 1995; 36:93–97.
[22] Storm FK, David MD, Mahvi M, Gilchrist KW. Heat shock protein 27 overexpression in breast cancer lymph node metastasis. Ann Surg Oncol. 1996; 3:570–573.
[23] Love S, King RJ. A 27 kDa heat shock protein that has anomalous prognostic powers in early and advanced breast cancer. Br J Cancer. 1994; 69:743–748.
[24] Assimakopoulou M, Bonikou GS, Maraziotis T, Varakis I. Prognostic significance of Hsp‑27 in astrocytic brain tumors: an immunohistochemical study. Anticancer Res. 1997; 17:2677–2682.
[25] King KL, Li AF, Chau GY. Prognostic significance of heat shock protein‑27 expression in hepatocellular carcinoma and its relation to histologic grading and survival. Cancer. 2000; 88:2464–2470.
[26] Ito T, Kawabe R, Kurasono Y, Hara M, Kitamura H, et al. Expression of heat shock proteins in squamous cell carcinoma of the tongue: an immunohistochemical study. J Oral Pathol Med. 1998; 27:18–22.
[27] Kawanishi K, Shiozaki H, Doki Y. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer. 1999; 85:1649–1657.
[28] Giaginis C, Daskalopoulou SS, Vgenopoulou S, Sfiniadakis I, Kouraklis G, et al. Heat shock protein‑27, ‑60 and ‑90 expression in gastric cancer: association with clinicopathological variables and patient survival. BMC Gastroenterol. 2009;9:14.
[29] Coffer AI, King RJ. Characterization of p29, an estrogen-receptor associated tumor marker. J Steroid Biochem. 1988; 31:745–750.
[30] Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, et al. Hsp27 overexpression inhibits doxorubicin‑induced apoptosis in human breast cancer cells. Breast Cancer Res Treat. 1999; 56:187–196.
[31] Kang SH, Kang KW, Kim KH, Kwon B, Kim SK, et al. Up‑regulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer. 2008; 8:286.
[32] Manish S, Umakant BP, Pravinkumar G, Anupama G. The significance of heat shock protein 27 in breast cancer: A signature to predict the outcome. Medical Journal of Babylon. 2023; 20:451–456.