Full Text
Introduction
Neonatal hyperbilirubinemia affects 8%-11% of neonates, with 60%-80% experiencing idiopathic jaundice. It is defined by total serum bilirubin (TSB) exceeding the 95th percentile during the first week of life [1]. Neonatal hyperbilirubinemia often requires phototherapy, occasionally exchange transfusion, and is more severe in preterm infants due to increased neurotoxicity risk [2].
Physiological jaundice occurs in newborns due to elevated bilirubin caused by increased red cell destruction and reduced hepatic conjugation. Excess bilirubin can exceed albumin's binding capacity, diffusing into extravascular tissues, including the central nervous system [3].
Severe jaundice can cause acute bilirubin encephalopathy with stupor and hypertonia, progressing to chronic encephalopathy, marked by athetosis, gaze abnormalities, sensorineural hearing loss, and rare intellectual deficit [4].
Neonatal jaundice is a leading cause of morbidity and mortality in newborns, affecting up to 60% of term and 80% of preterm infants. Early identification and monitoring of at-risk infants are crucial. While visual evaluation aids in detection, serum bilirubin measurement is the most accurate method for assessing severity [5, 6].
However, the blood test is an invasive method, stressful for parents and time consuming for staff [7]. Transcutaneous bilirubinometry (TcB) has been developed over the last two decades and is considered a non-invasive, convenient, and painless way of the estimating bilirubin level [8]. Bilirubin is measured by specific wave lengths of light directed into skin [9].
The American Academy of Pediatrics urges improved bilirubin measurement accuracy and further studies on the cost-effectiveness of transcutaneous methods [10]. Hyperbilirubinemia is defined as total serum bilirubin (TSB) levels exceeding the 95th percentile during the first week of life. Physiological jaundice, characterized by yellow discoloration of the skin due to bilirubin deposition, results from increased red cell destruction and immature hepatic bilirubin conjugation. Severe cases can lead to acute bilirubin encephalopathy, marked by neurological symptoms like stupor, hypotonia, and hypertonia. Chronic complications, such as extrapyramidal disturbances and sensorineural hearing loss, may develop if left untreated. Preterm infants are particularly vulnerable to bilirubin neurotoxicity due to lower thresholds for toxicity.
Hyperbilirubinemia contributes significantly to neonatal morbidity and mortality, affecting 60% of term and 80% of preterm neonates. Early identification of at-risk infants through clinical evaluation and monitoring is crucial. However, visual assessment of jaundice via skin and sclera inspection is subjective and influenced by factors such as skin color and hemoglobin levels. TSB measurement is the most reliable diagnostic method but requires invasive blood sampling, which is stressful for parents and time-intensive for healthcare providers.
To address these challenges, transcutaneous bilirubinometry (TcB) has emerged as a non-invasive, painless alternative for estimating bilirubin levels using specific wavelengths of light. TcB has demonstrated strong evidence as a reliable jaundice screening tool in term and late preterm infants. It has been included in the American Academy of Pediatrics (AAP) guidelines for managing hyperbilirubinemia in neonates with a gestational age of ≥35 weeks. However, TcB accuracy depends on factors like gestational age, skin color, birth weight, and phototherapy use [11].
Recent advancements in neonatal care have necessitated better bilirubin measurement tools due to shorter hospital stays and heightened vigilance regarding kernicterus risk. Serial TSB measurements are often required to monitor bilirubin levels and rate of change, which may involve multiple traumatic heel sticks. Suboptimal collection can result in hemolyzed samples, necessitating repeated sampling and causing additional distress. Despite the increasing adoption of TcB, its use is not yet widespread globally. The current study aimed to evaluate the correlation between TSB and bilirubin levels estimated by the AMIGO MBJ20 device, using forehead (TcB) and midsternum (TcBS) measurements, further validating TcB as a non-invasive method for neonatal jaundice assessment.
The study aimed to estimate serum bilirubin in babies using a non-invasive device (AMIGO MBJ20). It also seeks to examine the correlation between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB) measured on the forehead using this instrument.
Material and methods
The data source for this hospital-based observational study comprises neonates admitted to the Neonatal Intensive Care Unit (NICU) and receiving phototherapy in the Department of Paediatrics at ShrimatiHeeraKunwar Baa Mahila Hospital, Jhalawar. The study duration spans one year from February 2022 to January 2023, as approved by the Ethical Committee.
Inclusion criteria: Neonates admitted in Neonatal Intensive Care Unit in SHKBM Hospital receiving phototherapy for neonatal hyperbilirubinemia without any comorbidities with: gestational age >35 weeks, and weight more than 2kg.
Exclusion criteria: Neonate: babies who had received phototherapy previously, babies with major congenital malformations, babies with conjugated hyperbilirubinemia, babies with co-morbidities like birth asphyxia, septicemia, renal failure, and babies with birth weight < 2kg and gestational age < 35 weeks.
Data collection
This study was carried out in NICU of the Department of Pediatrics, Jhalawar medical college, during study period, Venous blood samples were collected from the neonates included in the study and sent for total bilirubin, direct bilirubin and transcutaneous bilirubin were measured by handheld bilirubinometer. Total and direct bilirubin was measured by Diazo method (Diazotized sulfanilic test).
The statistical methods used in this study include:
1. Pearson correlation coefficient: Used to assess the strength and direction of the linear relationship between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB). A strong positive correlation was found (r = 0.813, p < 0.001), indicating TcB can be a reliable surrogate for TSB.
2. Simple linear regression: The study derived a regression equation, TSB = 0.9987 × TcB - 1.6258, which allows for predicting TSB from TcB measurements with high accuracy.
3. Bland-Altman analysis: Used to evaluate the agreement between TSB and TcB measurements by determining the mean difference (-1.645) and limits of agreement (-5.972 to 2.682). This method confirmed that TcB slightly underestimates TSB on average.
4. Kendall’s coefficient of concordance (Kendall's W): Used to assess agreement between TSB and TcB, yielding a moderate agreement (Kendall's W = 0.595, p < 0.001).
5. ANOVA test: Used to compare mean bilirubin levels across different gestational age groups, with a p-value of 0.892, indicating no significant difference.
Results
The table 1 shows the distribution of postnatal age among 171 newborns, with 1.8% being 1 day old, 18.1% being 2 days old, and the majority (80.1%) aged 3 days or more Mean age in the study group was 3.53±1.28 days. Range is 1 to 8 days. Majority neonate’s age was 3+ days (80.1%).
Table 1: Postnatal age.
|
Postnatal Age
|
N
|
%
|
|
1 day
|
3
|
1.8
|
|
2 days
|
31
|
18.1
|
|
3+ days
|
137
|
80.1
|
|
Total
|
171
|
100
|
The table 2 shows the gestational age distribution of 171 newborns, with 15.2% born preterm (<37 weeks) and 84.8% born at term (37-42 weeks).the incidence of preterm babies was 15.2% (26). Mean gestational age in the study group was 37.49±1.15 weeks.
Table 2: Gestational age.
|
Gestational age
|
N
|
%
|
|
<37
|
26
|
15.2
|
|
37-42
|
145
|
84.8
|
|
Total
|
171
|
100
|
The table 3 shows the birth weight distribution of 171 newborns, with 16.4% weighing less than 2.5 kg (classified as low birth weight) and 83.6% weighing above 2.5 kg. Mean birth weight was 2.84±0.41 kg.
Table 3: Birth weight.
|
Birth weight
|
N
|
%
|
|
<2.5 kg
|
28
|
16.4
|
|
>2.5 kg
|
143
|
83.6
|
|
Total
|
171
|
100
|
The table 4 shows the total serum bilirubin levels for 171 newborns, with 33.3% having levels below 12 mg/dl, 61.4% falling between 12-18 mg/dl, and 5.3% exceeding 18 mg/dl.
Table 4: Total serum bilirubin distribution of babies.
|
Total serum bilirubin
|
N
|
%
|
|
<12mg/dl
|
57
|
33.3
|
|
12-18mg/dl
|
105
|
61.4
|
|
>18mg/dl
|
9
|
5.3
|
|
Total
|
171
|
100
|
The table 5 shows the distribution of transcutaneous bilirubin (TCB) values on the forehead in 171 newborns, with 64.9% having TCB levels between 12-18 mg/dl, 26.9% having levels below 12 mg/dl, and 6.4% exceeding 18 mg/dl.
Table 5: Transcutaneous bilirubin (Forehead) values distribution in babies.
|
TCB (Forehead)
|
N
|
%
|
|
<12mg/dl
|
46
|
26.9
|
|
12-18mg/dl
|
111
|
64.9
|
|
>18mg/dl
|
11
|
6.4
|
|
High
|
3
|
1.8
|
|
Total
|
171
|
100
|
The table 6 shows the total serum bilirubin (TSB) levels across different ages of babies, with the mean TSB ranging from 9.97 mg/dl at 1 day old to 16.80 mg/dl at 10 days old. The highest mean bilirubin levels were observed at 4 days old (14.30 mg/dl), while the variability, as shown by the standard deviation (SD), was relatively higher in some groups, such as the 2-day-olds (SD = 4.37). Overall, the mean TSB for the entire population was 12.99 mg/dl with a standard deviation of 3.79, indicating moderate levels of bilirubin across the sample, with some age groups showing more variation in values.
Table 6: Age of baby and total serum bilirubin.
|
Age of babies (In days)
|
N
|
Mean
|
SD
|
|
1
|
3
|
9.97
|
1.05
|
|
2
|
31
|
9.99
|
4.37
|
|
3
|
61
|
13.79
|
2.66
|
|
4
|
41
|
14.30
|
4.03
|
|
5
|
23
|
13.08
|
3.63
|
|
6
|
6
|
13.37
|
1.30
|
|
7
|
3
|
11.87
|
5.08
|
|
8
|
2
|
10.70
|
0.42
|
|
10
|
1
|
16.80
|
-
|
|
Total
|
171
|
12.99
|
3.79
|
Table 7 shows highest mean serum bilirubin value of 14.30 was seen in babies with a gestational age of 41 weeks. Lowest mean serum bilirubin value of 12.20 was seen in babies with a gestational age of 36 weeks.
Table 7: Gestational age and total serum bilirubin.
|
Gestational age(week)
|
N
|
Mean
|
SD
|
|
35
|
4
|
14.20
|
1.87
|
|
36
|
22
|
12.20
|
2.71
|
|
37
|
77
|
13.30
|
3.91
|
|
38
|
33
|
12.81
|
3.96
|
|
39
|
25
|
12.64
|
4.75
|
|
40
|
9
|
13.27
|
2.35
|
|
41
|
1
|
14.30
|
-
|
|
Total
|
171
|
12.99
|
3.79
|
Note: P= 0.892, Not-significant, ANOVA test.
The table 8 shows the Pearson correlation coefficient between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB) for 171 newborns, with a correlation coefficient (R) of 0.813. This strong positive correlation suggests that TSB and TcB measurements are closely related, indicating that TcB can be a reliable surrogate for TSB in assessing bilirubin levels. The p-value of <0.001 confirms that this correlation is statistically significant.
Table 8: Pearson Correlation Coefficient between TSB and TCB.
|
N
|
R
|
P value
|
|
171
|
0.813
|
<0.001
|
The table 9 shows the agreement between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB) measurements, based on the Bland-Altman plot analysis for 171 newborns. The mean difference between TSB and TcB was -1.645, with a standard deviation of 2.207, suggesting a slight underestimation of TcB compared to TSB on average. The limits of agreement ranged from -5.972 to 2.682, indicating that while most differences between TSB and TcB fall within this range.
Table 9: Agreement between TSB and TcB (by constructing Bland Altman plot).
|
|
N
|
Mean
|
SD
|
Lower limit
|
Upper limit
|
|
Difference of TSB and TCB
|
171
|
-1.645
|
2.207
|
-5.972
|
2.682
|
Figure 1 shows Simple linear regression is calculated as TSB = 0.9987 TCB -1.6258. This equation can be used for predicting TSB from TcB with a high degree of accuracy. X-axis suggests the mean of TSB and TcB. Y-axis was plotted by the difference between TSB and TcB. The bias line indicates a difference of 1.645 between the averages of the two variables. Analysis of mean bilirubin level showed that TSB was 1.645 lower than the measured TcB (Figure 1).
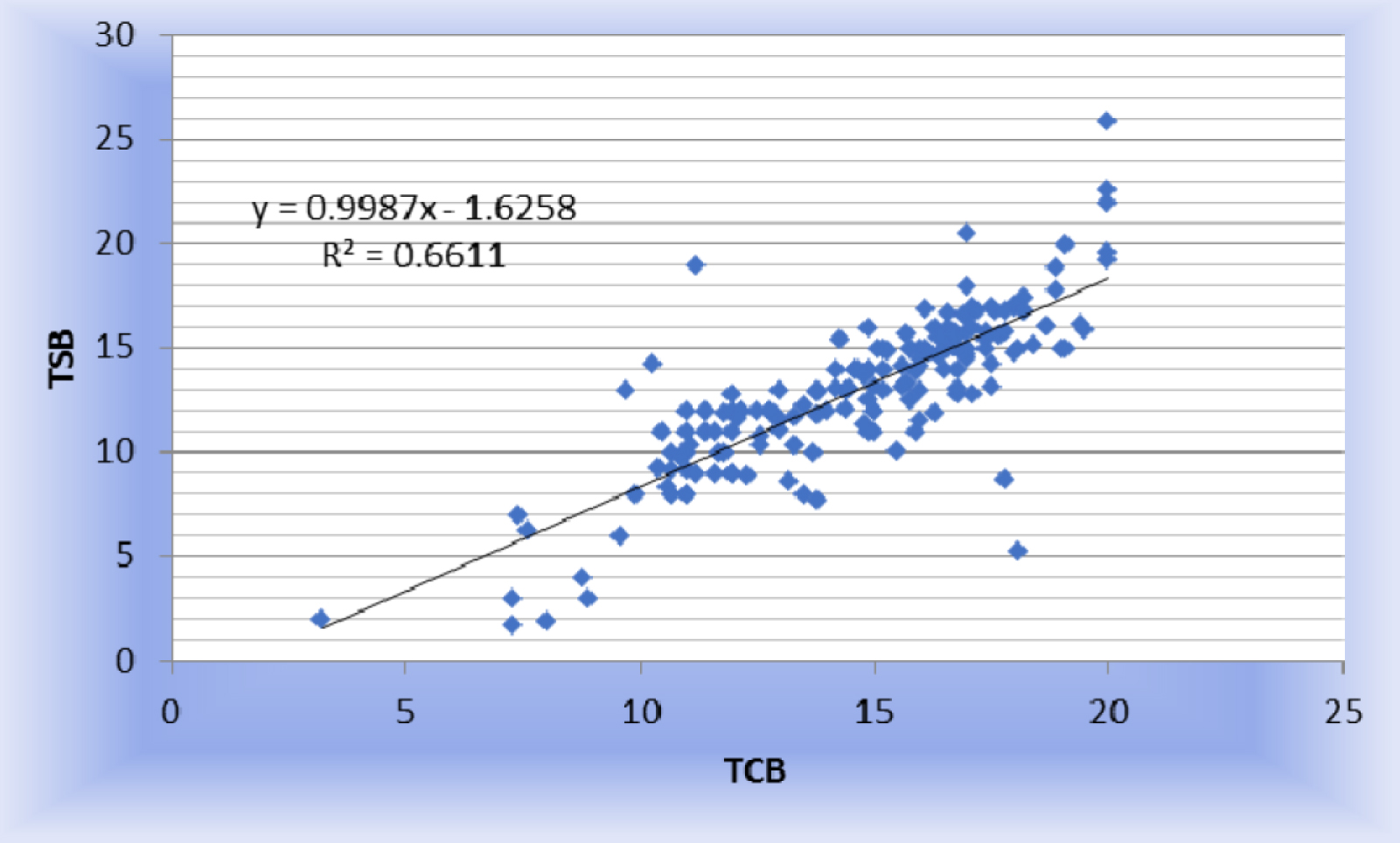
Figure 1: Scatter plot between TSB and TcB.
Figure 2 shows many of the data points fall within ±1.96 times the SD of the difference between the TSB and TcB values. This corroborates that there is a strong agreement between the TSB and TcB.
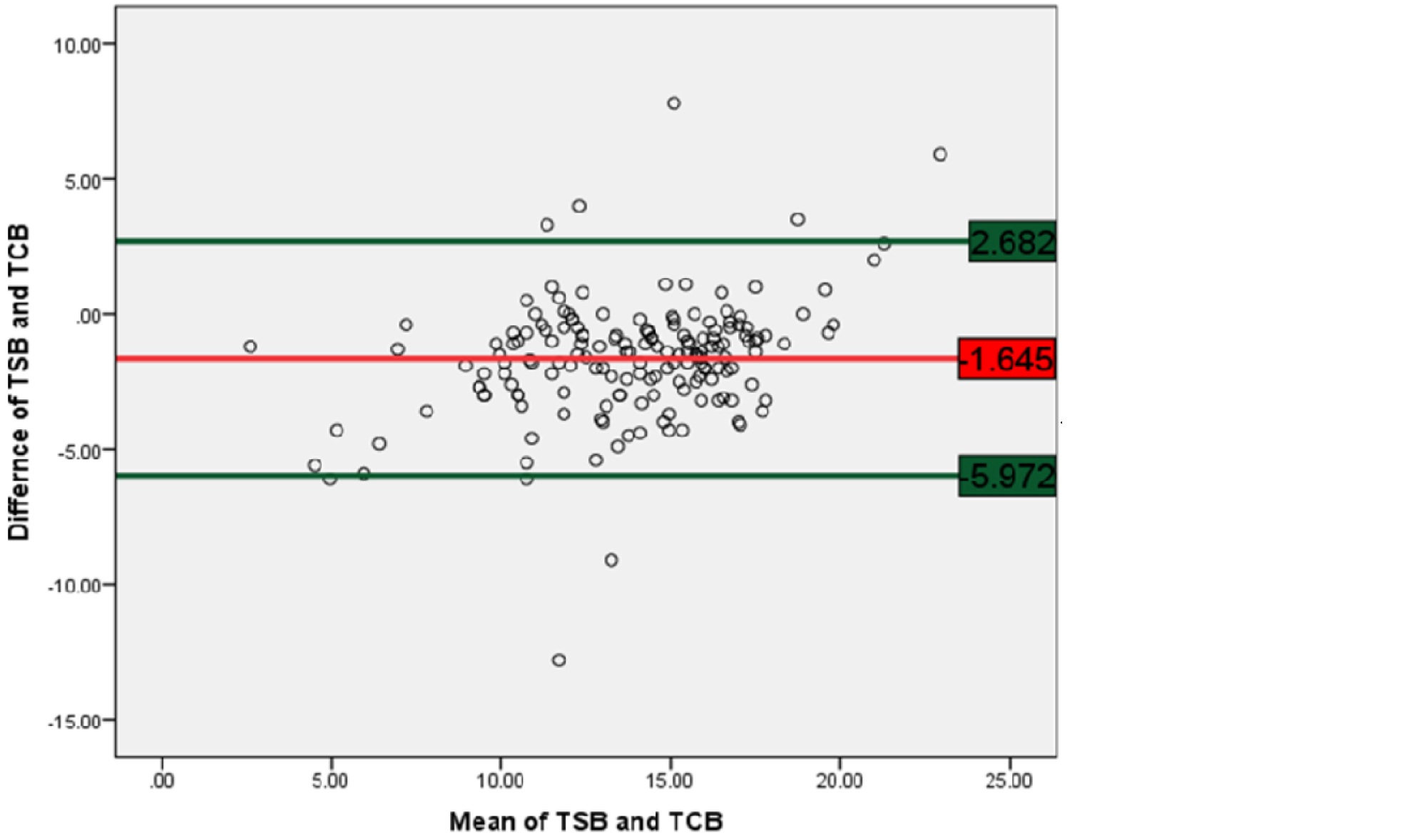
Figure 2: Bland-Altman plot of TSB versus TCB.
The table 10 shows the agreement between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB) using Kendall's Coefficient of Concordance for 171 newborns. The Kendall’s W value of 0.595 suggests a moderate level of concordance between the two methods, indicating that while TSB and TcB are generally in agreement, some degree of variation still exists. The chi-square value of 101.807 with a p-value of <0.001 further supports that the observed concordance is statistically significant.
Table 10: Agreement between TSB and TCB (by Kendall's Coefficient of Concordance).
|
N
|
Kendall's W
|
Chi-Square
|
P value
|
|
171
|
0.595
|
101.807
|
<0.001
|
Discussion
In our study only 4.7% neonates had APGAR scoreless than 7 at 1 minute after birth and none had APGAR scoreless than 7 at 5 minutes after birth. In a study done by Nlend et al [12] about 11.3% of the neonates had APGAR score below 7 at one minute after birth and 1.14% of neonates had APGAR score below 7 at five minutes after the birth. This is in similarity to the present study that most of the cases had good APGAR score at one minute and 5 minutes after birth. Comparison of basic demographic and clinical parameters are discussed in the table 11.
Table 11: Comparison with other studies.
|
Parameters
|
Present study
|
Study by Nlend et al [12]
|
Study by Keren et al [13]
|
|
Gender (male babies)
|
57.9%
|
55.68%
|
47.9%
|
|
Post natal age
|
|
-
|
|
1
|
1.8%
|
5.68%
|
|
|
2
|
18.1%
|
31.82%
|
|
|
>3 days
|
80.1%
|
62.5%
|
|
|
Birth weight
|
|
-
|
|
<2.5kg
|
16.4%
|
5.68%
|
|
|
2.5 to 4kg
|
83.6%
|
85.2%
|
|
|
>4kg
|
-
|
9.09%
|
|
|
Weight loss >5
|
|
-
|
|
<5%
|
21.6%
|
44.32%
|
|
| |
78.4%
|
55.68%
|
|
|
Gestational age
|
|
|
|
<37 weeks
|
15.2%
|
-
|
6.37%
|
|
37 to 42 weeks
|
84.8%
|
100%
|
93.6%
|
The threshold value of serum total bilirubin was 100 mg/l for diagnosis of hyperbilirubinemia suggested by the National Institute for Health and Care Excellence(NICE) guidelines. In the current study, the cut off value for hyperbilirubinemia is upto 12 mg/dl and 61.4% of the new-born’s included were having total serum bilirubin in the range of 12 to 18 mg/dl and nearly 5.3% of the newborns had total serum bilirubin above 18mg/dl. In study done [12], the mean total serum bilirubin was observed to be 123.8 ± 54.8 mg/l. Among 298 newborns included in this study, 6.82% of them were having total serum bilirubin above 20 mg/dl and 18.18% of the newborns had total serum bilirubin in the range of 15 to 20 mg/dl. There is similarity of such minor proportion of newborns having TsB>20 mg/dl with the present study. But the cut off values considered for both the studies were differing.
In our study, 64.9% had a TcB (Mid sternum) of 12 – 18 mg/dl, and 4.7% were having >18 mg/dl. 64.9% had a TcB (Forehead) of 12 – 18 mg/dl, while >18 mg/dl of TCB is seen in 6.4% of new-born’s included in the study. In study done by Nlend et al [12], the mean TcB (fore head) was observed to be 143.32 ± 45.31 mg/l. 28% of the neonates had TcB (forehead) within the range of 15 to 20 mg/dl while 12.50% of neonates had TcB (forehead) above 20mg/dl. The mean TcB (mid sternum) was observed to be 149.25 ± 47.64 mg/l. 31.82% of the neonates had TcB (forehead) within the range of 15 to 20 mg/dl while 11.36% of neonates had TcB(forehead) above 20mg/dl. Hence, similar trend of TcB at two anatomical sites is observed in the current study as well as the study done by Nlend et al [12].
In our study, we found statistically significant (p <0.001) strong positive linear correlation between TSB and TcB. Simple linear regression is calculated as TSB = 0.9987 TCB 1.6258. This equation can be used for predicting TSB from TcB with a high degree of accuracy.
In a study by Bell [14] correlation coefficient of serum total bilirubin and transcutaneous bilirubin values at mid sternal region was 0.609. The correlation coefficient of serum total bilirubin and transcutaneous bilirubin values at forehead was 0.707. Correlation coefficient of transcutaneous bilirubin levels measured at mid sternum, forehead was 0.732. these values are statistically significant (p value <0.001). In studies by Ho et al [15], Bhutani et al [16] and Kolmanet a [17], similar observations were made wherein, there was significant correlation between transcutaneous bilirubin and total serum bilirubin. A good correlation between transcutaneous bilirubin and total serum bilirubin was also observed by Juster-Reicher et al [18] (r value = 0.72). Another study by Panburana et al [19] observed a good correlation of transcutaneous bilirubin (TcB) with total serum bilirubin (TSB).
A strong agreement between the TSB and TcB is proved by Bland-Altman plot and“moderate” agreement is proved by using Kendall’s scale in the current study.
Nlend et al [12] found a correlation between the TcB of forehead and TcB of sternum r = 0.91 which is strong; Similar finding is seen in other study also (Chimhini et al [20]). Conversely, transcutaneous bilirubin levels on the forehead were found to have good correlation with total serum bilirubin in studies done by Oyapero et al [21], Mishra et al [22] and Kaynak-Turkmen et al [23]. The difference between mean levels of total serum bilirubin and transcutaneous bilirubin according to Bland and Altman method was found to be −29.68 mg/l in Kaynak-Turkmen et al [23] study. Such an inaccuracy was high when compared to findings of studies done by Oyapero et al [21] and Keren et al [13].
In a study done by Giuston et al [24] result shows in which correlation showed an area under the curve of 0.815 for TcBS, slightly higher than that obtained for TcBF, which was 0.813 for the cut-off point ≥ percentile 95.
In a systemic review by Charles Okwundu et al [24], 23 studies (5058 participants) that were conducted in different countries and settings, used different transcutaneous bilirubin measuring devices, and defined hyperbilirubinaemia with different bilirubin values. Some of the infants were premature and others were born at term (from 37 weeks' pregnancy); their ages ranged from birth to one month of life. Overall, the findings of the studies suggest that transcutaneous bilirubin measurement is a good screening tool for detecting hyperbilirubinaemia in newborns. The included studies found different degrees or levels of accuracy for the use of transcutaneous bilirubin measurement. However, due to the differences between studies, we could not provide an overall combined summary of the accuracy of the different tests. The differences in these studies included factors like the threshold values for hyperbilirubinaemia, the types of transcutaneous bilirubin measuring devices, and age and ethnicity/skin colour of the included infants.
The study observed a male incidence of 57.9% and female incidence of 42.1%, with a male-to-female ratio of 1.375:1. The mean age of neonates was 3.53 days, with 80.1% aged over 3 days. More than half of the mothers (53.2%) were primigravida. Preterm births were 15.2%, with a mean gestational age of 37.49 weeks. Normal deliveries accounted for 61.4%, while 4.7% of neonates had an Apgar score below 7 at 1 minute but none at 5 minutes. Low birth weight incidence was 16.4%, with a mean birth weight of 2.84 kg. Breastfeeding was initiated in 85.4% of cases. The most common maternal and neonatal blood group was O positive (43.9% and 36.3%, respectively).
Limitation of study: The data was collected from a tertiary care center with a limited patient sample, which may not accurately represent the community's disease profile. A multi-center study with a larger patient population would provide more precise and comprehensive results. The present study, based on a limited number of cases, is insufficient to draw conclusive findings.
Conclusion
Bilirubin levels showed that 61.4% of neonates had total bilirubin of 12-18 mg/dl, with a mean peak at day 10. Male and female mean bilirubin levels were 12.69 and 13.21, respectively. Significant correlation (p<0.001) was found between total serum bilirubin (TSB) and transcutaneous bilirubin (TcB), with a linear regression equation of TSB = 0.9987xTcB - 1.6258. Bland-Altman analysis confirmed strong agreement between TSB and TcB values, supported by Kendall's W of 0.595, indicating moderate agreement. The present study establishes agreement between TSB and TcB. “TSB = 0.9987xTCB-1.6258” equation can be used for predicting TSB from TcB with a high degree of accuracy.
Conflict of interest
Authors declare no conflict of interest.
References
[1] Ullah S, Rahman K, Hedayati M. Hyperbilirubinemia in neonates: types, causes, clinical examinations, preventive measures and treatments: a narrative review article. Iran J Public Health. 2016; 45:558–568.
[2] Ahmed M, Mostafa S, Fisher G, Reynolds TM. Comparison between transcutaneous bilirubinometry and total serum bilirubin measurements in preterm infants< 35 weeks gestation. Ann Clin Biochem. 2010; 47:72–77.
[3] Ahlfors CE. Criteria for exchange transfusion in jaundiced newborns. Pediatrics. 1994; 93:488–494.
[4] Connolly AM, Volpe JJ. Clinical features of bilirubin encephalopathy. Clinics in perinatology. 1990; 17:371–379.
[5] Slusher TM, Angyo IA, Bode–Thomas F, Akor F, Pam SD, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004; 113:1636–1641.
[6] Haque KM, Rahman M. An unusual case of ABO–haemolytic disease of the newborn. Bangladesh Med Res Counc Bull. 2000; 26:61–64.
[7] Maisels MJ. Managing the jaundiced newborn: A persistent challenge. Cmaj. 2015; 187:335–343.
[8] Hemmati F, Rad NA. The value of Bilicheck® as a screening tool for neonatal jaundice in the South of Iran. Iran J Med Sci. 2013; 38:122–128.
[9] Richard EL, Ammar RB, Tridente A, De Luca D. Relationship between transcutaneous bilirubin and circulating unbound bilirubin in jaundiced neonates. Early Hum Dev. 2016;103:235–239.
[10] American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004; 114: 297–316.
[11] Sankaran K. Transcutaneous bilirubinometry in neonates. Paediatrics & Child Health. 2006; 11:75–76.
[12] Nlend AEN, Ndjenje DK, Sandie AB. Correlation between transcutaneous bilirubinemia and blood bilirubinemia in screening term newborn for neonatal jaundice at the essos hospital centre (EHC), yaoundé, Cameroon. Open J Pediatrics. 2022; 12:594–605.
[13] Keren R, Tremont K, Luan X, Cnaan A. Visual assessment of jaundice in term and late preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009; 94:17–22.
[14] Bell FR. A study of correlation and agreement between transcutaneous bilirubinometry and serum bilirubin measurements in term neonates with clinical jaundice. 2015. Dissertation submitted to The Rajiv Gandhi University of Health Sciences, Bengaluru.
[15] Ho HT, Ng TK ,Tsui KC, Lo YC. Evaluation of a new transcutaneous bilirubinometer in Chinese newborns. Arch Dis Child Fetal Neonatal Ed. 2006; 91:34–38.
[16] Bhutani VK, Johnson LH, Keren R. Diagnosis and management of hyperbilirubinemia in the term neonate; for a safer first week. Pediatr Clin N Am. 2004; 51:843–861.
[17] Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007; 20:266–271.
[18] Juster–Reicher A, Flidel–Rimon O, RozinI, Shinwell ES. Correlation of transcutaneous bilirubinometry (tcb) and total serum bilirubin (tsb) levels after phototherapy. J Matern Fetal Neonatal Med. 2015; 28:1329–1331.
[19] Panburana J, Boonkasidach S, Rearkyai S. Accuracy of transcutaneous bilirubinometry compare to total serum bilirubin measurement. J Med Assoc Thai. 2010; S81–S86.
[20] Chimhini GL, Chimhuya S, Chikwasha V. Evaluation of transcutaneous bilirubinometer (DRAEGER JM 103) use in Zimbabwean newborn babies. Matern Health Neonatol Perinatol. 2018; 4:1.
[21] Oyapero O, Njokanma FO, Disu EA. Relevance of the jaundice meter in determining significant bilirubin levels in term neonates at a tertiary hospital in Lagos State. Archives Med Health Sci. 2018; 6:232–237.
[22] Mishra S, Chawla D, Agarwal R, Deorari A, Paul V, et al. Transcutaneous bilirubinometry reduces the need for blood sampling in neonates with visible jaundice. Actapaediatrica. 2009; 98:1916–1919.
[23] Kaynak–Turkmen M, Aydogdu SA, Gokbulut CS. Transcutaneous measurement of bilirubin in Turkish newborns: Comparison with total serum bilirubin. Turk J Pediatr. 2011; 53:67–74.
[24] Mendoza–Chuctaya G, Ramos–Chuctaya KR, Maraza–Aquino EJ, Ruiz–Esquivel JE, Velázquez–Córdova LAS. Accuracy of transcutaneous bilirubin measurement in full–term newborns at 3400 meters above sea level. Bol Med Hosp Infant Mex. 2021; 78:116–122.
[25] Okwundu CI, Olowoyeye A, Uthman OA, Smith J, Wiysonge CS, et al. Transcutaneous bilirubinometry versus total serum bilirubin measurement for newborns. Cochrane Database Syst Rev. 2023; 5:CD012660.