Orginal Research
2025
June
Volume : 13
Issue : 2
The impact of cyclin D1 on endometrial hyperplasia and endometrioid endometrial carcinoma
Sudha M, Shanmugapriya M, Vijay A
Pdf Page Numbers :- 90-95
Sudha M1, Shanmugapriya M2,*, and Vijay A3
1Department of Pathology, ACS Medical College and Hospital, Tamil Nadu 600077, India
2Department of Pathology, Meenakshi Medical College Hospital and Research Institute, Enathur, Tamil Nadu 631552, India
3Department of Pathology, St Peter's Medical College Hospital and Research Institute, Hosur, Krishnagiri District, Tamil Nadu 635130, India
*Corresponding author: Dr. Shanmugapriya M, Department of Pathology, Meenakshi Medical College Hospital and Research Institute, Enathur, Tamil Nadu 631552, India. Email: m.s.priya.85@gmail.com
Received 2 January 2025; Revised 8 March 2025; Accepted 17 March 2025; Published 24 March 2025
Citation: Sudha M, Shanmugapriya M, Vijay A. The impact of cyclin D1 on endometrial hyperplasia and endometrioid endometrial carcinoma. J Med Sci Res. 2025; 13(2):90-95. DOI: http://dx.doi.org/10.17727/JMSR.2024/13-16
Copyright: © 2025 Sudha M et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Endometrioid endometrial carcinoma (EEC) is the most common type of endometrial cancer, accounting for approximately 80-90% of all endometrial cancer cases. Endometrial hyperplasia deserves special attention because of its relationship with endometrialcinoma. This study's goal was to use immunohistochemistry to investigate the expression of cyclin D1 between hyperplasia without atypia, atypical hyperplasia and endometrioid endometrial carcinoma.
Methods: We evaluated and compared the expression of cyclin D1 in 15 endometrial samples, including 5 cases of hyperplasia without atypia, cases of atypical hyperplasia, and another 5 cases of endometrioid endometrial carcinoma.
Results: In our study results, cyclin D1 was considerably overexpressed in glands with endometrioid endometrial carcinoma compared to atypical hyperplasia and hyperplasia without atypia. Our investigation demonstrated a statistically significant relationship between diagnostic type and cyclin D1 expression levels (p-value = 0.034).
Conclusion: The current work demonstrates that cyclin D1 overexpression may be a useful biomarker for identifying subsets of endometrial lesions that are precancerous and hence amenable to surgical treatment. Cyclin D1 is a critical regulator of cell cycle progression and plays a significant role in the development and progression of endometrial carcinoma. Further research is needed to fully elucidate the mechanisms of cyclin D1 regulation and to develop novel therapeutic strategies targeting cyclin D1 and its regulatory mechanisms.
Keywords: cyclin D1; endometrioid endometrial carcinoma; endometrial hyperplasia; atypical hyperplasia; immunohistochemistry
Full Text
Introduction
Endometrial hyperplasia and endometrioid endometrial carcinoma (EEC) are significant health concerns for women, particularly those in their reproductive years. Cyclin D1 is a protein that plays a crucial role in cell cycle regulation, and its impact on these conditions is an area of active research [1, 2]. Cyclin D1 is essential for the transition from the G1 phase to the S phase of the cell cycle. When cyclin D1 is overexpressed, it can lead to increased cell proliferation and a higher risk of cancer development. Research indicates that cyclin D1 is often overexpressed in cases of endometrial hyperplasia and EEC. This overexpression can disrupt normal cell cycle regulation, contributing to tumor growth [3].
Studies indicate that there is a notable overexpression of cyclin D1 in cases of endometrial hyperplasia, which subsequently contributes to the abnormal growth of cells and the subsequent development of tumors. This particular overexpression can be attributed to a variety of factors, including hormonal influences, genetic mutations, and changes at the epigenetic level. The overexpression of cyclin D1 in endometrial hyperplasia holds considerable clinical relevance, as it serves not only as a diagnostic marker but also as a prognostic biomarker, indicating disease progression, and presents opportunities for potential therapeutic interventions [2, 3]. The implications of cyclin D1 overexpression in the context of endometrial hyperplasia are substantial, particularly concerning therapy. By targeting cyclin D1 with specific inhibitors or other therapeutic strategies, there is the possibility of developing a novel approach to treatment that could improve patient outcomes [4, 5].
This study aimed to comprehensively determine the intricate relationship between the expression levels of cyclin D1 and the various types of endometrial hyperplasia. The primary goal was to thoroughly explore the potential of cyclin D1 as a diagnostic marker that could effectively distinguish between these different hyperplastic lesions. Furthermore, this investigation sought to aid in the early detection of endometrial hyperplasia, particularly in instances where atypical characteristics are present.
Materials and methods
Fifteen endometrial samples were retrospectively collected from the pathology department, Meenakshi Medical college, Kancheepuram from March 2021 to February 2022 This study was approved by the Institutional Ethics Committee (IEC). Among these 15 samples selected, 5 cases were hyperplasia without atypia, 5 cases had features atypical hyperplasia, and another 5 cases were Endometrioid endometrial carcinomas. The specimens were examined grossly, and representative sections were obtained and processed using the routine procedures of dehydration, clearing, and embedding. Paraffin-embedded sections were cut to a thickness of 4 μm and stained with hematoxylin and eosin.
Inclusion criteria: Endometrial curettage and hysterectomy specimens with features of hyperplasia and EEC were selected.
Exclusion criteria: Cases with chronic non-specific endometritis, endometrial polyps, mixed patterns, fibroids, and endometrial curettages were excluded.
Cyclin D1 expression in endometrial hyperplasia was thoroughly analyzed employing the immunohistochemistry technique (pioneered by DAKO, located in Kyoto, Japan) in conjunction with the streptavidin-biotin method specifically applied to paraffin-embedded tissue sections. The analysis utilized breast carcinoma as a positive control to ensure the reliability and validity of the staining process. The intensity of nuclear staining was graded according to a defined scale consisting of four distinct categories: no staining (0), weak staining (1+), moderate staining (2+), and strong staining (3+). In addition to this intensity grading, the extent of staining was evaluated using a semi-quantitative method, which allowed for an assessment that ranged from 0% to 100%. This percentage was determined through meticulous counting of at least 50 nuclei. Following this count, the ratio of immunoreactive nuclei (those that exhibited positive staining) to the total number of nuclei present was calculated, and this ratio was subsequently multiplied by 100 to convert it into a percentage value. It was important to round these calculated percentages to the nearest 10% for clarity in reporting. In cases where less than 10% of the cells exhibited positive staining, a score of 0 was assigned to represent this lack of staining. If the positivity of cells fell within the range of 10% to 30%, this was assigned a score of 1. On the other hand, when the positivity rate was between 31% and 60%, a score of 2 was accordingly given. In instances where more than 60% of the cells were positive for staining, this was classified with a score of 3, indicating a strong presence of relevant immunoreactivity. Furthermore, a comprehensive statistical analysis was conducted using SPSS version 21.0 software (developed by IBM SPSS, located in the United States), which had the Regression Modules installed for advanced data evaluation. Notably, the results revealed a statistically significant association between the type of diagnosis and the levels of cyclin D1 expression, with a calculated p value of 0.034.
Results
Our study includes a total of 15 cases comprising 2 groups of hyperplasia [hyperplasia without atypia and atypical hyperplasia] and EEC. Each category accounts for 5 cases, representing 25% of the total cases (Table 1). Among the 15 cases, the 41–60 years age group had the majority of cases, with 7 cases accounting for 46.67% of the total. The >60 years age group follows, comprising 5 cases (33.33%), while the 25–40 years age group has the lowest representation, with 3 cases (20%). The overall study participants' ages ranged from 25 to 72 years. The mean age was 45 years (Table 2).
Table 1: Distribution of cases based on the histopathological diagnosis.
|
Diagnosis
|
Number of cases
|
Percentage (%)
|
|
Hyperplasia without atypia
|
5
|
25
|
|
Atypical hyperplasia
|
5
|
25
|
|
Endometrial endometrioid carcinoma
|
5
|
25
|
|
Total
|
15
|
100
|
Table 2: Distribution of cases across different age groups.
|
Age group (years)
|
Number of cases
|
Percentage (%)
|
|
25–40
|
3
|
20
|
|
41–60
|
7
|
46.67
|
|
>60
|
5
|
33.33
|
|
Total
|
15
|
100
|
Table 3 shows the cyclin D1 expression levels across different types of hyperplasia and endometrial carcinoma. In Hyperplasia Without Atypia, 80% of the cases were negative, and the remaining 20% of cases were positive for cyclin D1 (Figure 1c). For atypical hyperplasia, the majority (60%) were negative, with 20% showing 2+ (Figure 1d) and 20% showing 1+. In EEC, cyclin D1 expression varied, with 20% showing 1+, 40% showing 2+, and 20% showing 3+ expression (Figure 1f). The remaining 20% showed negative expression for cyclin D1.
Table 3: Cyclin D1 expression levels across different types of hyperplasia.
|
Diagnosis
|
Cyclin D1
|
Total
|
|
Negative
|
1+
|
2+
|
3+
|
|
Hyperplasia without atypia
|
4 (80%)
|
1 (20%)
|
0
|
0
|
5
|
|
Atypical hyperplasia
|
3 (60%)
|
1 (20%)
|
1 (20%)
|
0
|
5
|
|
Endometrial endometrioid carcinoma
|
1 (20%)
|
1 (20%)
|
2 (40%)
|
1 (20%)
|
5
|
Note: The scoring is based on the nuclear intensity and percentage of positive cells (16).
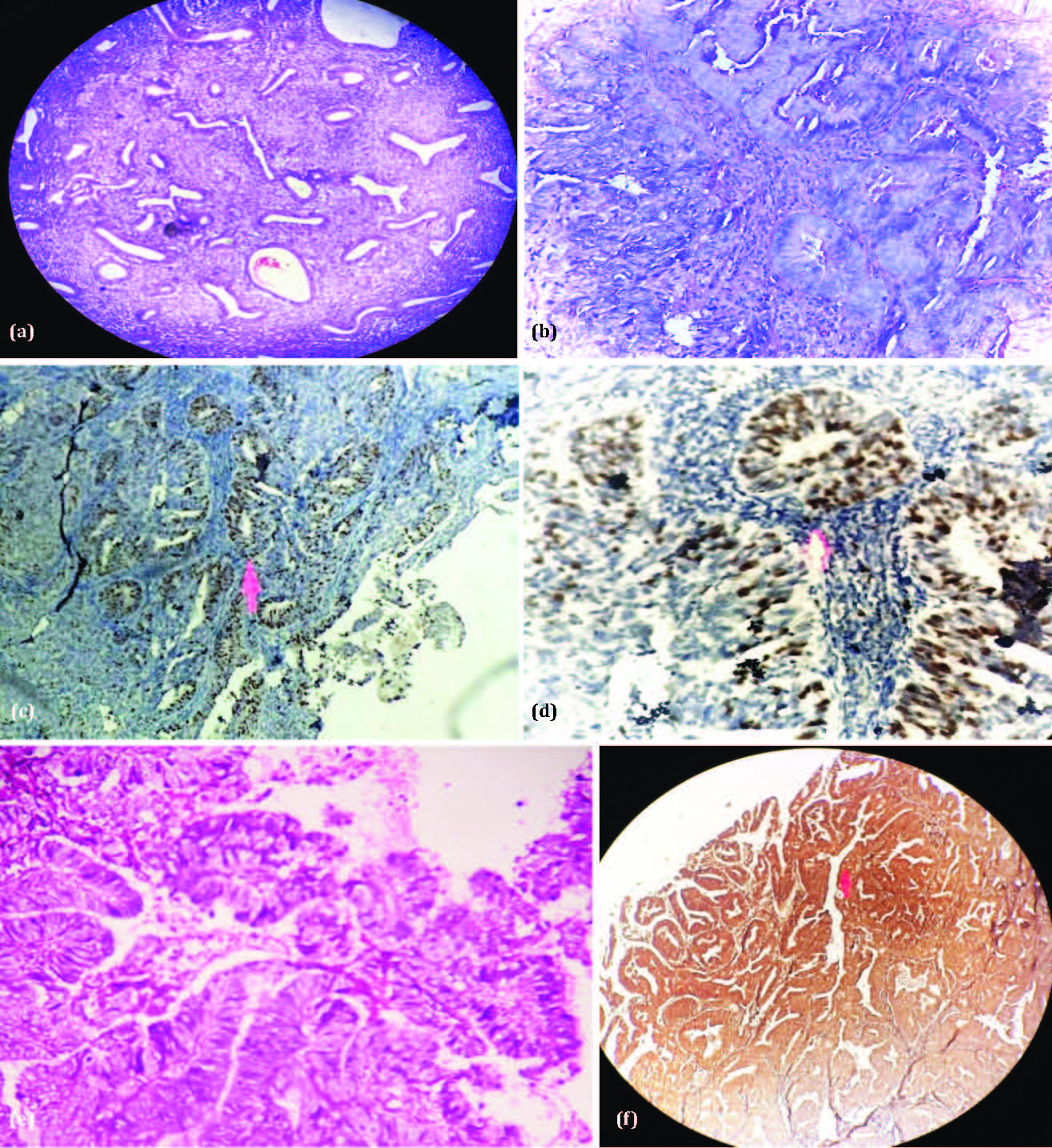
Figure 1: (a) Showing hyperplasia without atypia. (b) High-power view of hyperplasia with atypia and focal crowding. (c) Cyclin D1 expression in hyperplasia without atypia. (d) Cyclin D1 in atypical hyperplasia. (e) H&E showing endometrial carcinoma in high-power view(40X). (f) Showing strong staining for cyclin D 1 in EEC with Grade 3+.
Immunohistochemistry results of our current study show that there was a statistically significant association between the diagnosis type and the cyclin D1 expression levels (p-value = 0.034). The differences in cyclin D1 expression (negative, 1+, 2+, 3+) across the different types of hyperplasia and EEC were statistically significant (Table 4). The result of our study shows that in hyperplasia without atypia, 80% of cases are negative and 20% are positive for cyclin D1. In atypical hyperplasia, 60% of cases are negative, while 40% are positive. In EEC, the majority of the cases (80%) showed strong immunostaining for cyclin D1 expression. The Chi-square test reveals that the expression of cyclin D1 is significantly associated with hyperplasia and EEC with a higher expression seen in atypical hyperplasia and carcinomas (p value =0.002) (Table 5).
Table 4: Association between the cyclin D1 expression and hyperplasia.
|
Diagnosis
|
Cyclin D1
|
χ 2
|
Df
|
p-value
|
|
Negative
|
1+
|
2+
|
3+
|
|
Hyperplasia without atypia
|
4
|
1
|
0
|
0
|
13.67
|
6
|
0.034
|
|
Atypical hyperplasia
|
3
|
1
|
1
|
0
|
|
Endometrial endometrioid carcinoma
|
1
|
1
|
2
|
1
|
Table 5: Cyclin D1 expression with chi-square test.
|
Diagnosis
|
Cyclin D1 expression
|
χ 2
|
df
|
P value
|
|
Negative
|
Positive
|
|
Hyperplasia without atypia
|
4 (80%)
|
1(20%)
|
11.67
|
2
|
0.002
|
|
Atypical hyperplasia
|
3(60%)
|
2 (40%)
|
|
Endometrial endometrioid carcinoma
|
1(20%)
|
4 (80%)
|
Discussion
Cyclin D1 is a protein weighing 36 kDa and composed of 295 amino acids. It is mainly found in the nucleus, where it interacts with CDK4 and CDK6 to create active complexes. These complexes are responsible for phosphorylating and inactivating the retinoblastoma protein (Rb), facilitating the transition of cells from the G1 phase to the S phase of the cell cycle. Cyclin D1 plays a crucial role in cell cycle progression and is often described as a "restriction point" protein. It is necessary for the G1 to S phase transition and contributes to the regulation of cell growth, differentiation, and survival. Cyclin D1 is commonly overexpressed in various cancers, including those of the breast, lung, colon, and lymphoma. This overexpression can result in unchecked cell proliferation, tumor development, and metastasis. The increased levels of cyclin D1 may stem from gene amplification or transcriptional dysregulation in cancerous tissues. Our research utilizing immunohistochemistry has shown that cyclin D1 is overexpressed in atypical hyperplasia compared to hyperplasia without atypia, which are recognized as precursors to endometrioid adenocarcinoma [5-7].
As per Sangwan, Karuna, and colleagues' research, only 30% of simple hyperplasia cases showed positive results for cyclin D1, whereas 80% of complex hyperplasia cases showed positive results for cyclin D1. In our study, 40% of cases showed positivity for atypical hyperplasia. cyclin D1 positivity was found in 16 out of 20 cases of endometrial carcinoma, which was over 75%. Our study reveals similar findings with 80% positivity.
Quddus et al. [6] investigated the role of cyclin D1 in hyperplasia, finding cyclin D1 expression in 62% of simple hyperplasia cases, while 38% did not. In complex hyperplasia cases, 79% tested positive for cyclin D1, with only 21% showing negative results. These findings contrast with our current study, which shows 80% negativity for hyperplasia without atypia and positivity in only 20% of cases. Additionally, they reported that over 70% of endometrial carcinoma cases were positive for cyclin D1, which aligns with our findings.
In a study by Bansal et al. [7], increased cyclin D1 expression in a limited number of cases of hyperplasia without atypia, which correlates with our study results. The study by Qzuysal et al. [9] had different results. They found no cyclin D1 in simple hyperplasia. In complex atypical hyperplasia, only 7.1% and 26.6% with endometrial carcinoma were positive. This is much lower than our study. It is also lower than other reported studies. Brucka et al. [10] found no difference in immunoreactivity between complex and atypical hyperplasia, observing that it was enhanced when compared to normal endometrium. This result contrasts with our study, which shows a difference between atypical hyperplasia and hyperplasia without atypia. Choudhury et al. [11] found that cyclin D1 was not expressed in any cases of hyperplasia without atypia, whereas in our study it shows positivity in 20% of hyperplasia without atypia.These findings are not correlated with our study. In a study by Nishimura et al. [12], 25% tested positive for hyperplasia without atypia, which aligns with our findings.
Moreno-Bueno et al. [13] observed that only one out of 18 cases tested positive for atypical complex hyperplasia. Fang et al. [14] found 18% positive in endometrial atypical hyperplasia. Our study confirmed the presence of atypical endometrial hyperplasia across different grades, with forty percent of these cases showing a grade of 2+ and 1+. This finding aligns with the results of the above studies.
Cao et al. reported cyclin D1 overexpression in both endometrial hyperplasia and endometrioid carcinoma [15] and suggested cyclin D1 may act as an oncogene. The current study also shows overexpression of cyclin D1 in EEC compared to hyperplasia. This study's results are similar to our findings. Sangwan et al. [17] found that there is a statistical difference in cyclin D1 positivity between hyperplasia of the endometrium and EEC. Their study results showed overexpression of cyclin D1 expression in EEC than hyperplasia. This finding aligns with our research.
In a study by Szymaunkski et al. [18], cyclin D1 overexpression was observed in the early stages of EEC and complex hyperplasia, and proved cyclin D1 as an early biomarker for carcinogenic events. Our study also shows overexpression of cyclin D1 in EEC in various grades and supports the result of the above study. Therefore, more research is needed to explore the exact mechanisms. A better understanding of cyclin D1 regulation could lead to new treatments.
The limitations of a study utilizing immunohistochemical analysis exclusively must be recognized and addressed for a comprehensive understanding, and to enhance the robustness of the findings, it may be necessary to incorporate additional validation through alternative methodologies. This should include a thorough exploration of the underlying molecular mechanisms that are responsible for the overexpression of cyclin D1 in various pathologic conditions affecting the endometrium.
Conclusion
The identification of cyclin D1 as a potential biomarker for endometrial hyperplasia carries considerable clinical significance. By quantifying the levels of cyclin D1 in patients who are suspected of having endometrial hyperplasia, healthcare professionals can achieve a higher level of diagnostic accuracy and formulate more precise and tailored treatment strategies that are specific to each patient's condition. It is essential to conduct further research to substantiate the utility of cyclin D1 as a reliable biomarker, as well as to design and implement targeted therapeutic approaches that specifically focus on this protein in order to effectively manage endometrial hyperplasias and endometrial carcinoma. Therefore, the role of cyclin D1 in the context of endometrial hyperplasias is of immense importance, and the continuation of research efforts in this domain offers substantial potential for enhancing the processes of diagnosis and treatment for these medical conditions in the foreseeable future.
Conflicts of interest
The authors declare no conflicts of interest.
References
[1] Shawana S, Kehar SI, Masood S, Aamir I. Immunoexpression of cyclin D1 and PTEN in various endometrial pathologies. J Coll Physicians Surg Pak. 2016; 26:277–282.
[2] Wu W, Slomovitz BM, Soliman PT, Schmeler KM, Celestino J, et al. Correlation of cyclin D1 and cyclin D3 overexpression with the loss of PTEN expression in endometrial carcinoma. Int J Gynecol Cancer. 2006; 16:1668–1672.
[3] La Perle KM. Endocrine system. In: Maronpot RR, Boorman GA, Gaul BW, et al., editors. Pathology of Genetically Engineered and Other Mutant Mice. 2021; 16:355–377.
[4] Khabaz MN, Abdelrahman AS, Butt NS, Al-Maghrabi B, Al-Maghrabi J. Cyclin D1 is significantly associated with stage of tumor and predicts poor survival in endometrial carcinoma patients. Ann Diagn Pathol. 2017; 30:47–51.
[5] Sangwan K, Garg M, Pathak N, Bharti L. Expression of cyclin D1 in hyperplasia and carcinoma of endometrium and its correlation with histologic grade, tumor type, and clinicopathological features. J Lab Physicians. 2020; 12:165–170.
[6] Quddus MR, Latkovich P, Castellani WJ, Sung CJ, Steinhoff MM, et al. Expression of cyclin D1 in normal, metaplastic, hyperplastic endometrium and endometrioid carcinoma suggests a role in endometrial carcinogenesis. Arch Pathol Lab Med. 2002; 126:459–463.
[7] Tchakarska G, Sola B. The double dealing of cyclin D1. Cell Cycle. 2020; 19(2):163–178.
[8] Shevra CR, Ghosh A, Kumar M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J Postgrad Med. 2015; 61:15–20.
[9] Özuysal S, Öztürk H, Bilgin T, Filiz G. Expression of cyclin D1 in normal, hyperplastic and neoplastic endometrium and its correlation with Ki-67 and clinicopathological variables. Arch Gynecol Obstet. 2005; 271:123–126.
[10] Brucka A, Bartczak P, Ratyńska M, Sporny S. Immunohistochemical pattern of protein P21, cyclin D1 and cyclin E in endometrial hyperplasia. Pol J Pathol. 2009; 60:19–25.
[11] Choudhury M, Bansal S. Expression of cyclin D1 in endometrial hyperplasia and endometrial carcinoma. Indian J Pathol Microbiol. 2007; 50:708–710.
[12] Nishimura Y, Watanabe J, Jobo T, Kato N, Fujisawa T, et al. Cyclin D1 expression in endometrioid-type endometrial adenocarcinoma is correlated with histological grade and proliferative activity, but not with prognosis. Anticancer Res. 2004; 24:2185–2192.
[13] Moreno-Bueno G, Rodríguez-Perales S, Sánchez-Estévez C, Marcos R, Hardisson D, et al. Molecular alterations associated with cyclin D1 overexpression in endometrial cancer. Int J Cancer. 2004; 110:194–200.
[14] Nan F, Lü Q, Zhou J, Cheng L, Popov VM, et al. Altered expression of DACH1 and cyclin D1 in endometrial cancer. Cancer Biol Ther. 2009; 8:1534–1539.
[15] Cao QJ, Einstein MH, Anderson PS, Runowicz CD, Balan R, et al. Expression of COX-2, Ki-67, cyclin D1, and P21 in endometrial endometrioid carcinomas. Int J Gynecol Pathol. 2002; 21:147–154.
[16] Liang S, Mu K, Wang Y, Zhou Z, Zhang J, et al. Cyclin D1, a prominent prognostic marker for endometrial diseases. Diagn Pathol. 2013; 8:138.
[17] Sangwan K, Garg M, Pathak N, Bharti L. Expression of cyclin D1 in hyperplasia and carcinoma of endometrium and its correlation with histologic grade, tumor type, and clinicopathological features. J Lab Physicians. 2020; 12:165–170.
[18] Szymański M, Jerka D, Bonowicz K, Antosik P, Gagat M. Assessment of Cyclin D1 Expression: Prognostic Value and Functional Insights in Endometrial Cancer: In Silico Study. Int J Mol Sci. 2025; 26:890.