Orginal Research
2024
September
Volume : 12
Issue : 3
A study on neutrophilic versus eosinophilic predominance in chronic rhinosinusitis patients
Suresh A, Cheruvu SC, Lakshmanasamy H, Sunitha M, Hassan RFM
Pdf Page Numbers :- 189-194
Abinayaah Suresh1,*, Saranya Chithra Cheruvu1, Haribalan Lakshmanasamy1, Sunitha M1 and Rukhaiya Fatima Mohamed Hassan1
1Department of ENT, Sri Muthukumaran Medical College and Research Institute, Mangadu, Chennai – 600069, India
*Corresponding author: Dr. Abinayaah Suresh, MBBS; MS (ENT), Sri Muthukumaran Medical College Hospital and Research Institute, Chikkarayapuram, Mangadu, Chennai – 600069, India. Email: abin004doc@gmail.com.
Received 29 March 2024; Revised 4 June 2024; Accepted 11 June 2024; Published 18 June 2024
Citation: Suresh A, Cheruvu SC, Lakshmanasamy H, Sunitha M, Hassan RFM. A study on neutrophilic versus eosinophilic predominance in chronic rhinosinusitis patients. J Med Sci Res. 2024; 12(3):189-194. DOI: http://dx.doi.org/10.17727/JMSR.2024/12-36
Copyright: © 2024 Suresh A et al. Published by KIMS Foundation and Research Center. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Rhinosinusitis is defined as a multifactorial disorder with persistence of symptoms caused by inflammation of the sinonasal mucosa for 12 or more weeks with confirmation by diagnostic nasal endoscopy and computed tomography. The main aim of this study is to analyze the neutrophilic or eosinophilic predominance among CRS patients with histopathological examination for appropriate management, and to understand the association between the endotypes of CRS and blood parameters.
Materials and methods: A prospective cohort study was conducted on 137 patients over a period of two years at a tertiary centre. Diagnostic nasal endoscopy (DNE), computed tomography of the nose and paranasal sinus (CT PNS), complete haemogram (CBC), absolute eosinophil count (AEC), erythrocyte sedimentation rate (ESR), immunoglobulin E(IgE) was done. Patients were planned for functional endoscopic sinus surgery (FESS) and specimen was sent for histopathological examination.
Results: The comparison between DNE and AEC groups showed statistical significance and so did the comparison between CT and IgE. When we compared the groups of CRS with and without polyps with DNE and CT, there was no statistical significance. Also 64.2% of patients had an eosinophilic predominance.
Conclusion: It has also been reported that CRS are highly eosinophilic, also have substantial levels of neutrophils. Importantly, tissue neutrophilia has been associated with a poor response to corticosteroid therapy in patients with CRS with nasal polyps.
Keywords: nasal polyp; eosinophils; neutrophils; sinusitis; allergy; asthma; inflammation
Full Text
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the nose and the paranasal sinuses due to infections, allergy, certain syndromes relating to mucociliary clearance and the presence of metabolic diseases such as diabetes. Allergy and asthma being a unified airway disease is one of the major concerns when it comes to quality of life and management.
Rhinosinusitis in adults is defined as inflammation of the nose and the paranasal sinuses characterised by two or more symptoms, one of which should be either nasal blockage or obstruction or congestion , nasal discharge (anterior/ posterior nasal drip) with or without facial pain/pressure with or without reduction or loss of smell and or either endoscopic features of nasal polyps and/or mucopurulent discharge primarily from middle meatus and/ or oedema / mucosal obstruction primarily in middle meatus and/or computed tomography of the nose and paranasal sinus (CT PNS) mucosal changes in the osteomeatal complex and/or sinuses, according to European position paper on rhinosinusitis and nasal polyps (EPOS 2012). Difficult to treat rhinosinusitis is defined as patients who have persistent symptoms of rhinosinusitis despite appropriate treatment. As the phenotypes do not provide full insight of the underlying cellular and molecular pathophysiology identification of endotypes is ideal for therapy that can be targeted against the heterogenic pathophysiology for effective treatment and better patient outcomes [1-3]. EPOS2020 has included primary and secondary CRS and characterised them based on anatomic distribution into localized and diffuse disease. Diffuse CRS are predominantly classified as ECRS (Eosinophilic chronic rhinosinusitis) and non-ECRS, determined by histological quantification of eosinophils, i.e. number/high powered field as per the EPOS panel.
As EPOS 2020 has prioritised endotype evaluation in the treatment protocol for CRS and as there are very limited studies and data on the histological and biochemical markers of CRS, we have taken up this study. Importantly, tissue neutrophilia has been associated with a poor response to corticosteroid therapy in patients with CRS with nasal polyps [4].
The study aimed to analyze the predominance of neutrophils and eosinophils among chronic rhinosinusitis patients with histopathological examination for appropriate management of CRS and to understand the association between the endotypes of CRS and blood parameters.
Materials and methods
A prospective cohort study was conducted in the Department of ENT, Sri Muthukumaran Medical College and Research Institute, Mangadu for a period of two years from January 2022 and December 2023. 137 patients diagnosed with chronic rhinosinusitis based on EPOS 2012 were taken up for the study. Sample size was estimated by using nMaster software version 2.0 with an alpha of 0.05 (2 sided), precision level of 5% and desired confidence level 95 % the estimated sample size using the sample size method for single proportion test. Ethical Standards: This study was done adhering to strict ethical principles with prior clearance from the Institutional Ethics Committee. Informed consent was taken for all patients and procedure was explained in their own language.
Patients who were above 14 years of age and who consented for the study with a diagnosis of chronic rhinosinusitis with or without polyps, fungal sinusitis and recurrent polyps and who failed medical management were selected for the study. Patients with conditions such as vasomotor rhinitis, nasal tumors/malignancies and previously operated for nasal surgeries are excluded.
Procedure
The patients were routinely evaluated using diagnostic nasal endoscopy (DNE) and computed tomography of paranasal sinuses (CT PNS) as a part of the diagnostic criteria of chronic rhinosinusitis. A complete blood count (CBC), absolute eosinophil count (AEC), erythrocyte sedimentation rate (ESR) and IgE (Immunoglobulin E) was done for all the patients. Disease severity on CT PNS and DNE will be calculated using Lund Mackay scoring and Modified Lund Kennedy scoring respectively.
According to the European Position Paper on Rhinosinusitis and nasal polyps (EPOS), chronic rhinosinusitis is defined as a diagnosis made on clinical grounds based on the presence of characteristic symptoms, combined with objective evidence of mucosal inflammation for duration of 3 months. CRS is termed when the subject has presence of 2 primary symptoms or 1 primary and 1 additional symptom with either endoscopic or radiological evidence.
0-15 points for less severe disease and 16-24 for more severe disease. Modified Lund-Kennedy endoscopic grading which is a three-point scoring system (0=absent, 1=mild, 2=severe) was used to analyze the variables: edema, discharge and polyps. A combined score (right + left side) of 0-12 was given (Table 1).
Table 1: Modified Lund-Kennedy scoring as described by Alkis J. Psaltis et al [4].
|
Criteria for Assessment
|
0
|
1
|
2
|
|
Polyp
|
Absent
|
Limited to middle meatus
|
Extending to the nasal cavity
|
|
Discharge
|
Absent
|
Hyaline
|
Thick or mucopurulent
|
|
Edema
|
Absent
|
Mild/moderate
|
Polypoidal degeneration
|
Preoperative disease load was calculated by assessing the severity on a CT scan and scoring was based on Lund Mackay scores [5, 6] (Table 2). The disease severity was graded based on the opacification noted in each of the sinuses and the OMC (Osteomeatal complex). Grading of disease severity on CT scan was 0-15 points for less severe disease and 16-24 points for more severe disease.
Table 2: Lund Macay scoring as per Lund VJ, Mackay IS [5].
|
Sinus
|
Right
|
Left
|
|
Maxillary
|
0 / 1 / 2
|
0 / 1 / 2
|
|
Anterior ethmoid
|
0 / 1 / 2
|
0 / 1 / 2
|
|
Posterior ethmoid
|
0 / 1 / 2
|
0 / 1 / 2
|
|
Sphenoid
|
0 / 1 / 2
|
0 / 1 / 2
|
|
Frontal
|
0 / 1 / 2
|
0 / 1 / 2
|
|
Osteomeatal complex*
|
0 / 2
|
0 / 2
|
Note: 0 = no abnormalities, 1 = partial opacification, 2 = complete opacification 0* = not occluded, 2* = occluded (mentioned in table also).
Mucosa from middle meatus in cases of CRS without polyp, polyps in cases of CRS with polyps and pus and tissue for fungal sinusitis patients were sent as specimen samples in patients who underwent functional endoscopic sinus surgery for histopathological examination to evaluate the number of eosinophils and neutrophils per high power field (with 40x magnification) cells per high power field were calculated and results were tabulated.
Eosinophilic predominance or neutrophilic predominance or mixed variants have been categorized and compared to the phenotypical findings elicited in CT PNS and DNE and also analysis was done in comparison with the blood parameters for appropriate treatment protocol.
The collected data was analysed with IBM, SPSS statistics software 20.0 Version. To describe about the data descriptive statistics, frequency analysis, percentage analysis were used for categorical variables and for continuous variables the mean and S.D was used. To find the association of significance in categorical data the Chi-Square test was employed. In all the above statistical tools the probability value < 0.05 was considered as significant level.
Results
Out of the total of 137 patients, 16 patients were less than 20 years of age (11.7%), 50 were aged between 21-40 (36.5%), 61 patients were between 41-60 (44.5%) and 10 patients aged above 60 (7.3%) (Figure 1).
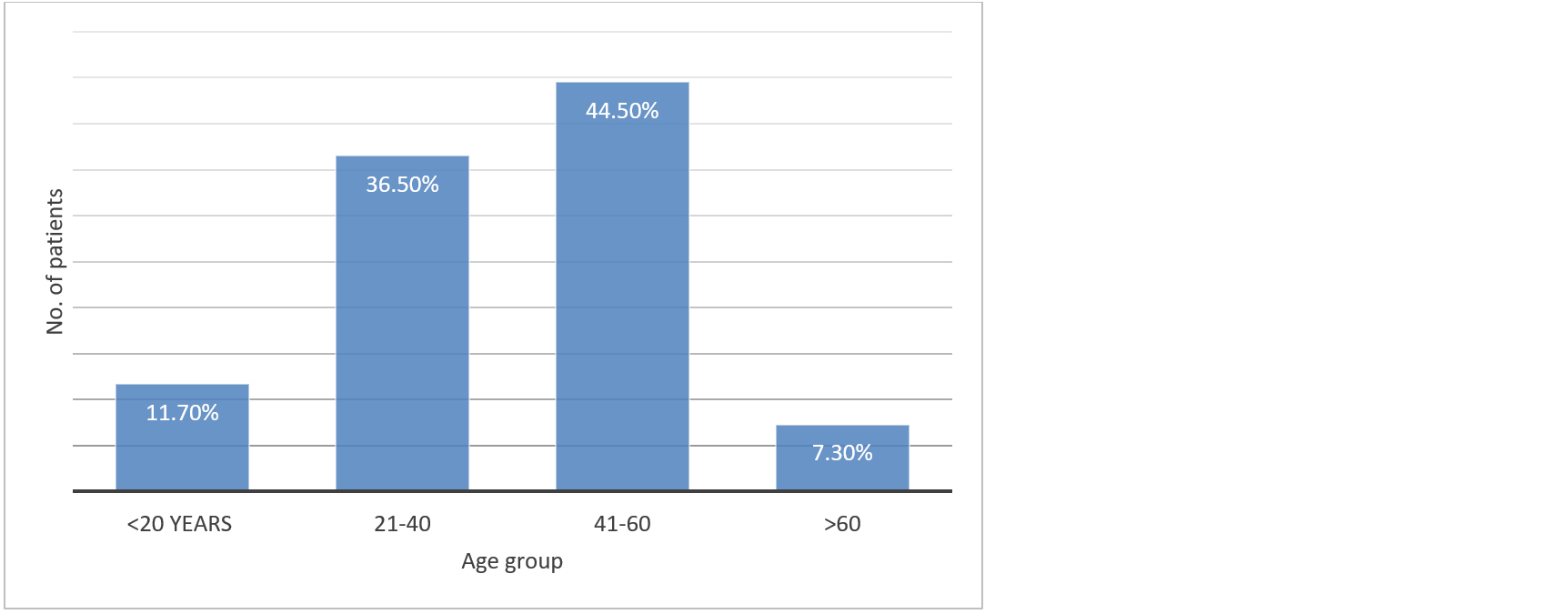
Figure 1: Prevalence of CRS based on age groups.
Out of 137, 132 (96.4%) patients experienced nasal obstruction, 116 patients (84.7%) had symptoms of headache, 33 (24.1%) patients came with complaints of facial pain and 126 (92%) had discharge in the middle meatus (Figure 2).
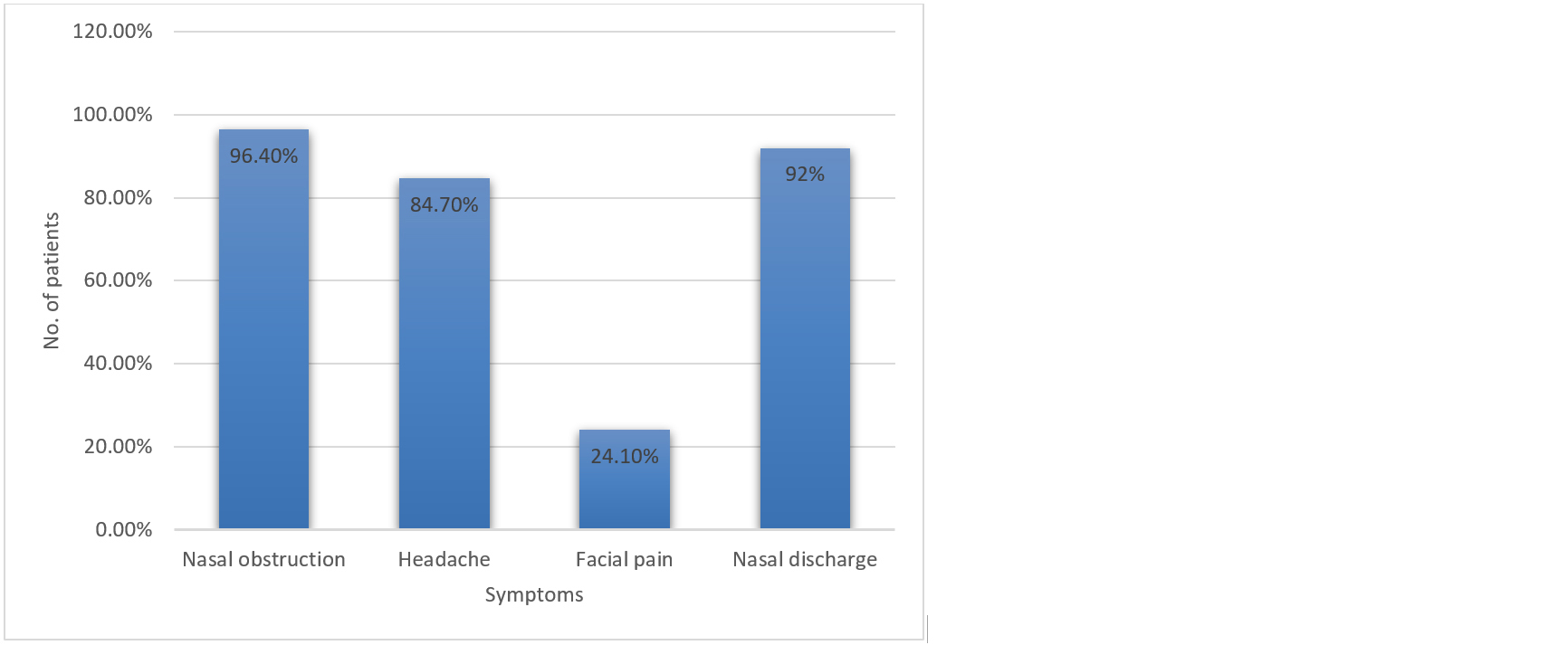
Figure 2: Presence of nasal symptoms associated with CRS.
There is significant correlation between DNE Modified Lund Kennedy scoring and Lund Macay scoring (out of 24) for CT at p < 0.001. There is significant correlation between DNE Modified Lund Kennedy scoring and AEC 30-350 Cells/ML at p > 0.05. There is significant correlation between DNE Modified Lund Kennedy scoring and IgE at p > 0.05 as per Spearmans rho tests (Table 3).
Table 3: Showing correlation between DNE and CT scorings along with AEC and IgE.
|
Spearman's RHO
|
Modified Lund Kennedy
scoring for DNE
|
Lund macay scoring
(out of 24) FOR CT
|
AEC 30-350 Cells/ML
|
IgE
|
|
DNE modified Lund Kennedy scoring
|
| |
Correlation Coefficient
|
1
|
0.282**
|
0.058
|
0.163
|
| |
Sig. (2-tailed)
|
0
|
0.001
|
0.5
|
0.057
|
| |
N
|
137
|
137
|
137
|
137
|
|
Lund Macay scoring
|
| |
Correlation Coefficient
|
0.282**
|
1
|
0.168*
|
0.234**
|
|
(out of 24) for CT
|
| |
Sig. (2-tailed)
|
0.001
|
0
|
0.05
|
0.006
|
| |
N
|
137
|
137
|
137
|
137
|
|
AEC 30-350 Cells/ML
|
| |
Correlation Coefficient
|
0.058
|
0.168*
|
1
|
0.177*
|
| |
Sig. (2-tailed)
|
0.5
|
0.05
|
0
|
0.039
|
| |
N
|
137
|
137
|
137
|
137
|
|
IgE
|
| |
Correlation Coefficient
|
0.163
|
0.234**
|
0.177*
|
1
|
| |
Sig. (2-tailed)
|
0.057
|
0.006
|
0.039
|
0
|
| |
N
|
137
|
137
|
137
|
137
|
Note: **= Correlation is significant at the 0.01 level (2-tailed); *= Correlation is significant at the 0.05 level (2-tailed).
There is no significant difference between CRS with polyp and without polyp in DNE Modified Lund Kennedy scoring at p > 0.05.
There is significant difference between CRS with polyp and without polyp in Lund Macay scoring (out of 24) FOR CT at p < 0.01. There is no significant difference between CRS with polyp and without polyp in ESR at p > 0.05. There is significant difference between CRS with polyp and without polyp in IgE at p < 0.001(Table 4).
Table 4: Showing correlation between DNE and CT scorings along with ESR and IgE.
| |
Modified Lund Kennedy scoring
(out of 12) for DNE
|
Lund Macay scoring
(out of 24) for CT
|
ESR (mm/hr)
|
IgE
|
|
Mann-Whitney U
|
1962.5
|
1592
|
2091.5
|
1346
|
|
Wilcoxon W
|
3288.5
|
2918
|
3417.5
|
2672
|
|
Z
|
-1.113
|
-2.757
|
-0.458
|
-3.773
|
|
Asymp. Sig. (2-tailed)
|
0.266
|
0.006
|
0.647
|
0
|
Samples sent for HPE examination were analysed and segregated into eosinophilic, neutrophilic and mixed types. Figure 3a&b showed eosinophilic infiltration and neutrophilic infiltration respectively. In our study 26.3% showed neutrophilic predominance, 64.2% showed eosinophilic predominance and mixed in 9.5%.
The mean and standard deviation values for the neutrophil/ eosinophil/mixed variety are shown in table 5. There is high eosinophilic predominance seen from the sample collected in our study.
Table 5: Mean and standard deviation values for DNE and CT scoring in relation to neutrophil/ eosinophil/mixed variety.
| |
N
|
Mean
|
Std.
Deviation
|
Std.
Error
|
95% Confidence
Interval
for Mean
|
Minimum
|
Maximum
|
|
Lower bound
|
Upper bound
|
|
Modified Lund Kennedy scoring
|
| |
Neutrophil
|
36
|
8.44
|
1.796
|
0.299
|
7.84
|
9.05
|
6
|
12
|
|
For DNE
|
| |
Eosinophil
|
88
|
8.86
|
2.403
|
0.256
|
8.35
|
9.37
|
6
|
12
|
| |
Mixed
|
13
|
8
|
2
|
0.555
|
6.79
|
9.21
|
6
|
12
|
| |
Total
|
137
|
8.67
|
2.227
|
0.19
|
8.3
|
9.05
|
6
|
12
|
|
Lund Macay scoring
|
| |
Neutrophil
|
36
|
10.56
|
5.649
|
0.941
|
8.64
|
12.47
|
4
|
24
|
|
(out of 24) for CT
|
| |
Eosinophil
|
88
|
12.7
|
6.497
|
0.693
|
11.33
|
14.08
|
4
|
24
|
| |
Mixed
|
13
|
11.38
|
4.857
|
1.347
|
8.45
|
14.32
|
4
|
20
|
| |
Total
|
137
|
12.01
|
6.181
|
0.528
|
10.97
|
13.06
|
4
|
24
|
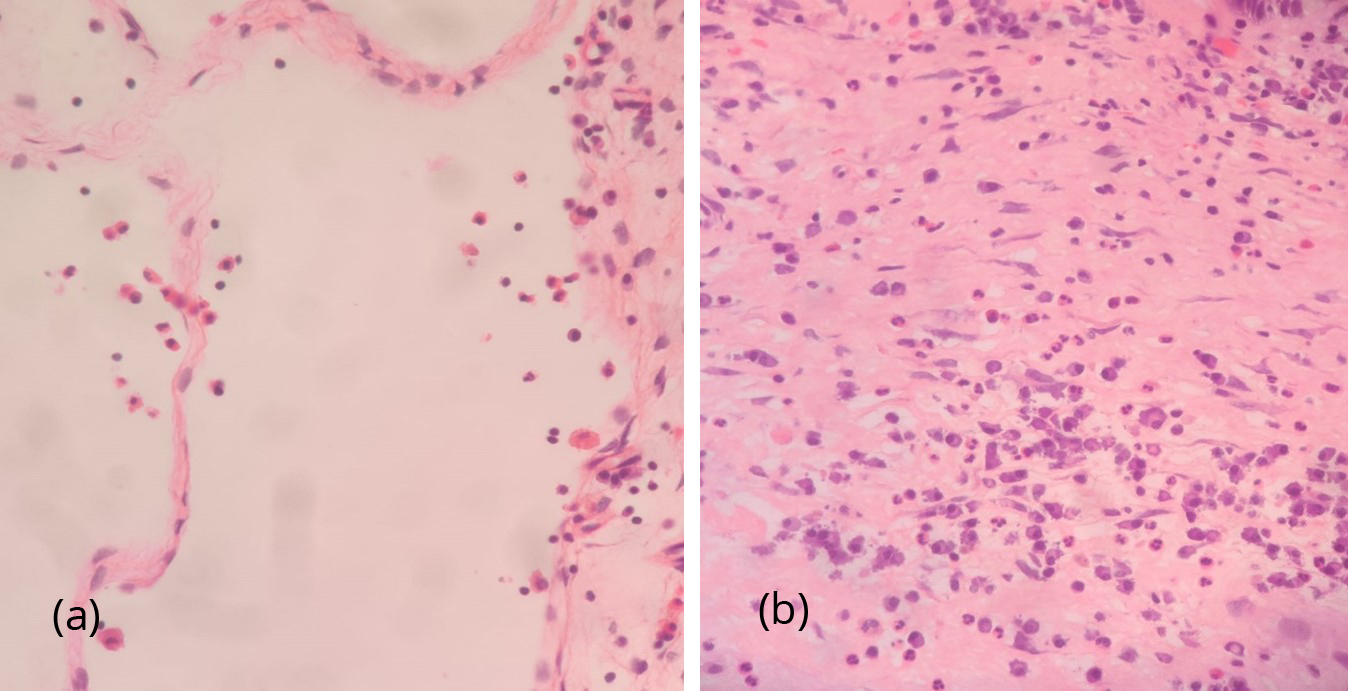
Figure 3: (a) HPE with 40x magnification showing predominantly eosinophilic infiltration (25-30 eosinophils/hpf) with scattered lymphoplasmacytes. (b) HPE with 40x magnification showing nasal polyp showing dense inflammatory infiltrates composed of neutrophils and lymphoplasmacytes.
Discussion
Chronic rhinosinusitis is the most common condition worldwide, leading to significant burden on healthcare and productivity. Chronic rhinosinusitis affects about 5-12% of the general population [7].
The epidemiology varies between type 2 and non-type 2 inflammation depending on various risk factors such as socioeconomic status, occupational exposure, habits like smoking, metabolic disorders such as diabetes, associated asthma or allergy along with certain genetic or hereditary factors [8]. Such varied combination of risk factors makes this condition quite challenging in some patients as there is always a risk of recurrence. High levels of eosinophilic markers and Th2 polarization with high interleukins IL-5, IL-13 are significantly present in CRSwNP (Chronic rhinosinusitis with nasal polyps) patients, whereas Th1 polarization with high levels of IFN-γ and TGF-β are seen in CRSsNP (Chronic rhinosinusitis without nasal polyps) [9].
In our study 62.8% of subjects had polyps, 64.2% had polyps with eosinophilic predominance whereas 26.3% had neutrophil dominance and 9.5% had a mixed variety. 37.2% of subjects did not have any polyps which is similar to the above said studies.
In countries like Japan people diagnosed with CRS belonged to the non-eCRS category previously before World war but after 1990’s the trend changed and majority of people were categorized as the eCRS variety. This can be attributed to various exposures related to industrialization and causes unknown leading to an increase in comorbidities such as allergy and asthma [9]. In a study by Staikuniene et al 69.4% presented with nasal polyps, 39.6% cases had a corelation with asthma and 45.5% with allergic rhinitis. Almost 91.7% of cases were linked with both asthma and nasal polyps has a synergistic effect both allergy and asthma need to be addressed being a unified airway disease [10].
In the west a study conducted among 376 patients only 19 patients (5.05%) had diabetes mellitus whereas India being the diabetic capital of the world, our study shows the prevalence of Diabetes Mellitus and CRS among our subjects was estimated about 37% [11, 12].
Around 60-90 % of CRSwNP patients in the western population has been eosinophil dominant variety. In a study conducted in India by Guthikonda MR et al, the prevalence was around 74.6% of eCRS and 23.6% of non eCRS among the total cases [11]. Tissue eosinophilia and systemic eosinophilia is seen predominantly in eCRS cases whereas tissue eosinophilia is absent in non eCRS. Patients show resistance to corticosteroids in non eCRS and also less prone to loss of smell [13].
Recalcitrant to therapy is seen in 23-50% of patients with CRS and recurrence due to deficiency of immunoglobulin IgA is seen in 13% [14,15]. Short course of oral corticosteroids is recommended to be more effective in eCRSsNP than intranasal corticosteroids [16]. Few studies have recommended low salicylate diet which has significant improvement in quality of life and symptomatology of CRS [17,18]. Emerging usage of immunomodulators in treatment of CRS considers the evidence of type 2 inflammation with tissue eosinophilia more than 10/HPF or blood eosinophilia more than 250 or total IgE levels more than 100 [19].
Traditional concepts of eosinophilia had changed due to newer concepts relating to cellular functions and there has been more focus on eosinophil targeted therapies at the molecular level [20]. The advantage of biomarkers is that they are non-invasive and helps us to diagnose a particular disease even before the clinical manifestations, however they lack specificity [21].
Limitations of the study: Investigations pertaining to the biomarkers of CRS and long term follow up in mixed histopathology i.e., Eosinophilic and neutrophilic CRS was difficult to assess for the refractiveness or recurrence of the disease.
Conclusion
Our study shows a wide eosinophil dominance among our patient group which is in sync with most other studies. However, the numbers are slightly higher owing to the large population. Such patients with eosinophilic predominance will benefit from antihistamine along with intranasal corticosteroid sprays and oral therapies whereas the role of antibiotics might not be beneficial. The understanding of the immune mechanism involved in CRS and categorising it into eCRS and non-eCRS has given promising results in the treatment protocols for recalcitrant CRS patients and also treatment protocols are patient centric based on their underlying pathology where they can receive targeted therapies. Recommendations to conduct further studies in biomarkers related to endotypes and also biological treatment for neutrophilic CRS will be helpful.
Conflicts of interest
Authors declare no conflicts of interest.
References
[1] Anderson GP. GR Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008; 372:1107–1119.
[2] Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–360.
[3] Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012; 67:835–846.
[4] Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. 2020; 58:82–111.
[5] Psaltis AJ, Li G, Vaezeafshar R, Cho KS, Hwang PH. Modification of the Lund- Kennedy endoscopic scoring system improves its reliability and correlation with patient‐ reported outcome measures. The Laryngoscope. 2014; 124:2216–2223.
[6] Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993; 31:183–184.
[7] Loos DD, Lourijsen ES, Wildeman MAM, Freling NJM, Wolvers MDJ, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol. 2019; 143:1207–1214.
[8] Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of chronic rhinosinusitis: prevalence and risk factors. J Allergy Clin Immunol Pract. 2022; 10:1395–1403.
[9] Zele TV, Claeys S, Gevaert P, Maele GV, Holtappels G, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289.
[10] Staikūnienė J, Vaitkus S, Japertienė LM, Ryškienė S. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Medicina. 2008; 44:257.
[11] Guthikonda MR, Gude A, Nutakki A. Eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps and their clinical comparison in Indian population. Indian J Otolaryngol Head Neck Surg. 2022; 74:994–1000.
[12] Zhang Z, Adappa ND, Lautenbach E, Chiu AG, Doghramji L, et al. The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. Int Forum Allergy Rhinol. 2014; 4:315–320.
[13] Grayson JW, Hopkins C, Mori E, Senior B, Harvey RJ. Contemporary classification of chronic rhinosinusitis beyond polyps vs no polyps. JAMA Otolaryngol. Neck Surg. 2020; 146:831–838.
[14] Schwitzguébel AJP, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J. Allergy Clin. Immunol. 2015; 136:1523–1531.
[15] Mazza JM, Lin SY. Primary immunodeficiency and recalcitrant chronic sinusitis: A systematic review. Int Forum Allergy Rhinol. 2016; 6:1029–1033.
[16] Miechowski W, Czerwaty K, Godlewska I, Dżaman K. Atopy as a specific predictor of response to systemic and local steroid therapy in patients with chronic rhinosinusitis without nasal polyps. Otolaryngol. Polska. 2022; 76:26–31.
[17] Sowerby LJ, Patel KB, Schmerk C, Rotenberg BW, Rocha T, et al. Effect of low salicylate diet on clinical and inflammatory markers in patients with aspirin exacerbated respiratory disease—A randomized crossover trial. J. Otolaryngol Head Neck Surg. 2021; 50:27.
[18] Sommer DD, Hoffbauer S, Au M, Sowerby LJ, Gupta MK, et al. Treatment of aspirin exacerbated respiratory Disease with a low salicylate diet. Otolaryngol Neck Surg. 2014; 152:42–47.
[19] Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; 58:1–464.
[20] Jesenak M, Diamant Z, Simon D. Eosinophils- from cradle to grave. Allergy. 2023; 78:3077–3102.
[21] Seah JJ, Thong M, Wang Y. The diagnostic and prognostic role of biomarkers in chronic rhinosinusitis. diagnostics (Basel). 2023; 13:715.