Abstract
Background: Although surgical excision is still the gold standard treatment for ocular surface squamous neoplasia (OSSN), interest in conservative medical approaches is steadily growing due to its advantages. The clinical outcome of topical mitomycin C (MMC) as the primary treatment for OSSN was reported. The primary outcome measure was clinical regression of the tumour. The secondary Outcome measures were duration of treatment, possible side effects and recurrences.
Materials and methods: In this prospective study 11 patients with the diagnosis of OSSN were included. All were treated with mitomycin C eye drops 0.02% 4 times daily for 4 days a week. The study was conducted at Mysore Medical College & Research Institute, Mysore for a period of 6 months from August 2020 to January 2021.
Results: Out of the 11 patients, complete tumour regression was achieved in 6 (54.5%) patients. The remaining 5 patients reported partial tumour regression. Mean duration of treatment was 6 weeks. The most common side effect noted was eye irritation. The side effect was transient and resolved with the cessation of treatment. No recurrence was observed during the follow up.
Conclusion: Topical chemotherapy can be tried as primary treatment in all patients with OSSN as it can cause complete or partial tumour regression with negligible side effects.
Keywords: ocular surface squamous neoplasia; mytomycin C; tumour regression
Full Text
Introduction
Ocular Surface Squamous Neoplasia (OSSN) is the most common malignancy of the ocular surface. It encompasses wide spectrum of histological diagnoses ranging from mild intraepithelial dysplasia to invasive squamous cell carcinoma (SCC) of the conjunctiva and cornea [1-12].
The most widely accepted treatment strategy for OSSN includes wide excision biopsy with 3–4 mm tumour- free margin by “no touch” technique for the conjunctival component, alcohol treated limited superficial keratectomy for the corneal component, followed by adjunct double freeze thaw cryotherapy to the conjunctival surgical margins and surface reconstruction [1-4, 8, 13-16]. However surgical excision has a recurrence rate ranging from 5% to 69% [1, 5-8, 13-15, 17, 18]. The other disadvantages of surgical excision include the risk of limbal stem cell deficiency, ocular surface irregularity and scarring, forniceal shortening, symblepharon and limitation of extra ocular movements [2, 7, 17, 19]. Hence the preferred treatment modality for OSSN is slowly shifting from surgery to topical chemotherapy [1, 6, 7, 13, 14]. While in dysplasia and carcinoma in situ it may help in complete resolution, it may also be useful in cases of SCC for chemoreduction [3].
Advantages of topical treatment for OSSN are simplicity of the treatment and avoidance of the surgical complication such as limbal stem-cell deficiency and corneal scarring [1, 7, 13]. The greatest advantage is that it treats the entire conjunctival surface with less dependence on defining tumor margins [14, 20]. This is beneficial, especially, in cases of multifocal tumours and subclinical dysplasia [1, 7, 13, 14, 21]. It also delivers high concentration of drug to the target tissue and spares surrounding normal tissue which is otherwise excised during surgery [14]. Unlike surgical excision the topical treatment can be titrated and even repeated according to the clinical response. But the long term effects of use of these topical agents on ocular surface are not known [14]. The tumour resolution is not immediate and hence the number of follow up visits required with medical treatment is more compared to primary surgery [4, 13]. The greatest limitation for use of topical therapy is the side effect profile and hence the compliance of the patient [6, 14]. However these side effects are self-limiting and resolve once the treatment is stopped [4].
Despite its advantages, there is paucity of published literature on efficacy of topical chemotherapeutic agents as primary treatment of OSSN. Moreover there is no consensus or guideline as to the best management protocol for OSSN [1, 14]. This study has been done to determine the efficacy of one of the topical chemotherapeutic agents mitomycin C (MMC) as primary treatment for OSSN.
Investigation was done the clinical outcome of topical MMC as the primary treatment for OSSN. The secondary Outcome measures were duration of treatment, possible side effects and recurrences.
Materials and methods
This study was approved by Institutional Ethical Committee. All cases of OSSN who presented to our hospital during the study period were included in the study. The study was conducted at Mysore Medical College & Research Institute, Mysore for a period of 6 months from August 2020 to January 2021. A written consent was taken from each patient. The diagnosis of OSSN was based on clinical findings. A detailed history was taken and complete ophthalmological examination was done. The demographic details (age, gender) were taken. Presenting complaints and duration of symptoms were noted. Risk factors and previous treatment history, if present, were recorded. The tumour characteristics like tumor laterality, tissue involved (conjunctiva, cornea, conjunctival limbal corneal surface), tumor location (superior, nasal, inferior, temporal quadrants; upper tarsus, lower tarsus, upper fornix, lower fornix), maximum basal tumor diameter (mm), number clock hours of limbal involvement, tumor pattern (leukoplakic, gelatinous, nodular, papillary, placoid, pigmented) clinically detectable multifocality, presence of feeder vessel and keratin were documented. Locoregional lymph node examination and medical examination to rule out locoregional or systemic metastasis of tumor were performed in all cases. Patients were also evaluated for presence of any systemic predisposing conditions.
All patients were treated with topical chemotherapy with 0.02% MMC administered 4 times a day for 4 days a week [9]. Patients were instructed about the possible local side effects. Topical chemotherapy course was repeated in those cases which showed response until 2 weeks after complete clinical tumour regression. A complete tumour regression was defined as total disappearance of lesion with clear visibility of underlying structures [22]. Those cases which showed no evidence of tumour regression were taken up for surgical excision. Patients were followed up every week while on topical chemotherapy and monthly thereafter, upto 6 months. Tumour details were recorded during each visit. Ocular side effects and any event of tumor recurrence were noted during follow-up.
Patients who were noncompliant to treatment, or those who were lost to follow up were excluded from the study.
Windows SPSS version 20 was used for all statistical analyses, and a p value 0.05 was considered statistically significant.
Results
Eleven eyes of eleven patients were included in the study. The mean age at presentation was 55.82years 18.3years (range: 28-76 years). There was male preponderance with male to female ratio of 7:4 (Figure 1). History of prior intervention was present in 1 patient. History of prolonged exposure to sunlight was noted in 5 patients (45%) and history of smoking in 3(27.3%) (Table 1). None of these demographic factors had statistically significant correlation with OSSN, (p>0.05). 2 patients had HIV. Of the 2, one was diagnosed to have HIV during evaluation of OSSN. The ages of the 2 patients with HIV and OSSN were 28 and 40 years, which was significantly lesser than the mean age of the patients with OSSN and no HIV. This association was found to be statistically significant (p=0.05).
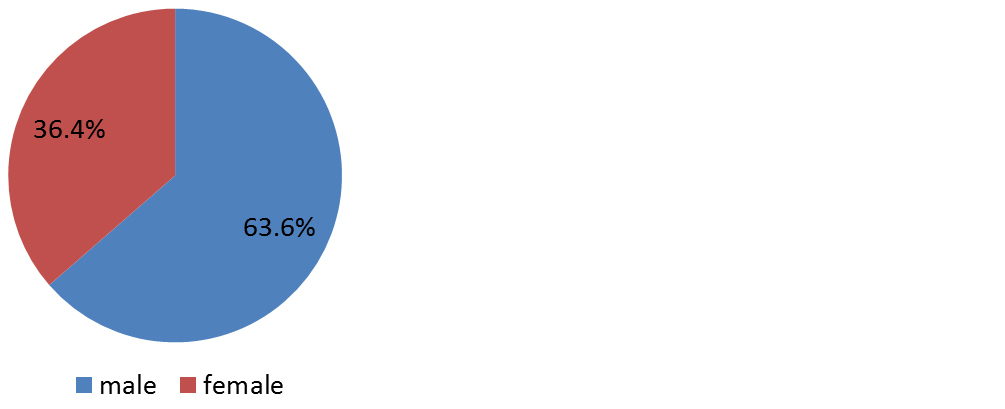
Figure 1: Gender distribution.
Table 1: Risk factors for OSSN.
|
Risk factors
|
Frequency
|
Percentage
|
|
HIV
|
2
|
18.2%
|
|
Smoking
|
3
|
27.3%
|
|
UV exposure
|
5
|
45.5%
|
|
Nil
|
1
|
9%
|
The most common presenting complaint was mass on the ocular surface. The mean duration of symptoms was 5.73 months (median: 4months; range: 1 month – 12 years).
The tumor involved conjunctivo-limbal corneal surface in 9(81.8 %), and bulbar conjunctiva in 2(18.2%). The mean basal dimension of the tumor was 5.73 mm (median: 5 mm; range: 4–10 mm). The mean number of limbal clock hours involved by the tumor was 3.89 (median: 2; range: 2-9). Most common clinical type noted was Leukoplakic, which was seen in 6 patients followed by gelatinous in 4 patients and papilliform in 1 patient (Table 2). The common location was temporal part (7/11).
Table 2: Clinical types of OSSN.
|
Type of lesion
|
Number
|
Percentage
|
|
Leukoplakic
|
6
|
54.5%
|
|
Gelatinous
|
4
|
36.4%
|
|
Papilliform
|
1
|
9.1%
|
|
Total
|
11
|
100%
|
Complete tumor regression by topical treatment alone was achieved in 6(54.5%) patients (p=0.05). Partial tumour resolution was seen in 5 patients (Figure 2). The mean duration for complete tumour regression was 6 weeks (p=0.03).
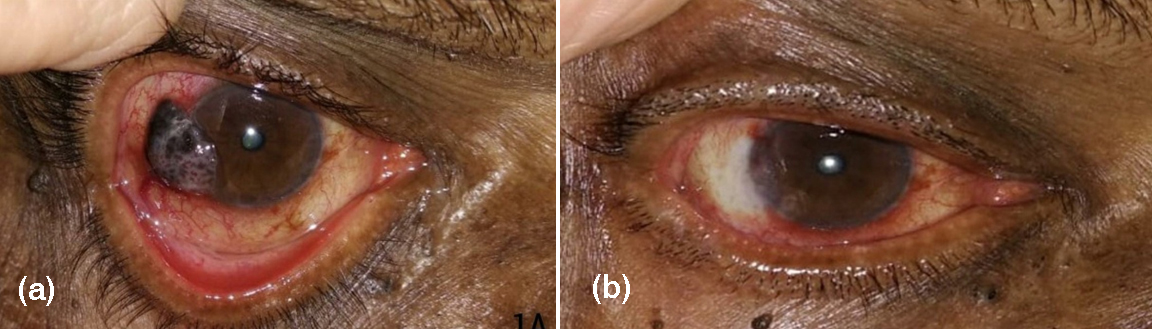
Figure 2: Before (a) and after (b) treatment with MMC.
Eye irritation was the common side effect noted (p=0.34). This was managed with carboxymethyl cellulose 0.5% eye drops and loteprednol 0.5% eye drops. The side effects were transient and resolved with the discontinuation of topical chemotherapy (Figure 3). No cases of punctual stenosis or corneal ulceration were observed. No patient discontinued therapy due to side effects. No tumor recurrence was noted in any patient.
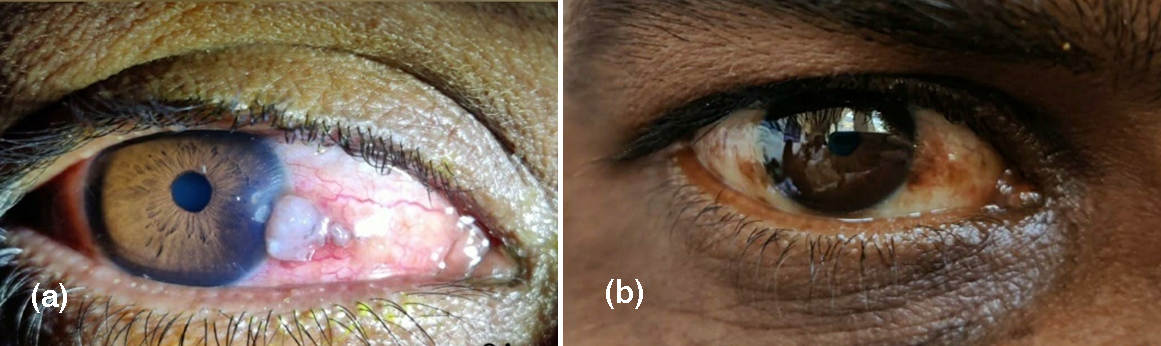
Figure 3: Before (a) and after (b) treatment with MMC.
Discussion
Despite the advantages of topical chemotherapy in the treatment of OSSN there is paucity of published articles on that report the clinical effects of these drugs. Our study describes the outcome of topical MMC in the treatment of OSSN. In our study complete tumour regression with topical treatment alone was achieved in 54.5% of the patients after a mean duration of 4 weeks. The remaining patients also showed partial tumour reduction leading to the reduction in the size of the tumour thus avoiding the complications related to the extensive surgical excision. Hence the topical treatment had proved to be beneficial in all patients. The efficacy rate of topical MMC in treatment of OSSN is ranged between 70% and 100% in the previous studies [13, 14, 23, 24]. Gupta and Muecke used topical MMC as primary treatment in diffuse conjunctival corneal intra-epithelial neoplasia and found that topical MMC is effective in 87.5% [13]. Meel et al also reported complete tumour regression in 87.5% of the cases and partial regression in the remaining 12.5% of the cases on treatment with topical MMC [8].
Most of the studies follow the treatment regimen of 1 week on and 1 week off of topical MMC 0.04% for treatment of OSSN [9, 13, 14, 17, 22, 24]. In our study topical MMC 0.02% was used in a pulsed manner with 4 days on and 3 days off treatment regimen. This improves the patient compliance by reducing the side effects. This also prevents damage to more slowly dividing epithelial cells and limbal stem cells, allowing them to repair their DNA [6, 17].
Demographic profile of patients with OSSN differ in different parts of the world [5]. The average age of presentation is usually the sixth and seventh decades of life. However in patients with systemic predisposing conditions HIV infection, xerodermapigmentosa, OSSN may occur at a younger age [1, 8, 17, 19]. According to the previous studies OSSN is commonly seen in elderly and in males in Asia, Europe, Australia and North America, but not in Africa [2, 5, 8, 11, 24, 25]. In Africa prevalence is more in young and females [5, 8, 11, 24], this may be due to increased prevalence of HIV infection in Africa. Generally male preponderance is seen as men are exposed to more UV light due to more outdoor activities. 2,5 Demographic profile of patients in our study was consistant with other studies from East Asia in having male preponderance and common age of presentation being sixth decade [5, 25].
Exposure to UV radiation in the form of excess outdoor activity was the most common risk factor noted in our study. UV radiation is the most common mutagenic factor implicated in pathogenesis of OSSN in many other studies [1, 2, 9, 22]. UV radiation causes p53 gene mutation thereby leading to OSSN [17].
HIV has been associated with eightfold increased risk of OSSN [2, 10]. OSSN can be the first presenting sign of HIV/ AIDS in 50–86% of cases [2]. One of the patients included in our study was diagnosed to have HIV infection during evaluation of risk factors for OSSN.
Similar to other studies, the lesions were most commonly located at limbus [2, 8, 22]. In our study leukoplakic type was the most common type. Another study from India by Kusumesh R et al also found that leukoplakic was most common type. A study from Thaiwan by Ma et al., found that papilliform type was more common [5]. Meel et al., in their study on clinicodemographic profile of patients with OSSN found gelatinous type more common [8].
MMC has been reported to cause conjunctival injection, irritation, pain, punctual stenosis, and periocular dermatitis [1, 2, 18, 19, 26-28]. These side effects are transient and resolved once the treatment was stopped. They can be easily managed with topical steroid and lubricant drops. MMC is also associated with limbal stem cell deficiency with keratopathy and with the reactivation of herpes simplex virus type keratitis [1, 21, 29, 30]. These side effects are rare when MMC is used in pulsed manner, as used in this study. In our study side effects were seen in 72.7% (8/11 patients).The most common side effect seen was eye irritation. This symptom was managed with loteprednol eye drops 0.5% and carboxymethyl cellulose 0.5% eye drops. Poothullil and Colby [28] reported the presence of clinically detectable side effects in 76% of patients treated with 0.04% MMC. In the largest study which included 58 patients to review the outcome and complications of MMC in the treatment of OSSN, allergic reaction and punctal stenosis were the main complications noted [16].
The mean duration required for tumour resolution was 4 week. This comparable with the result of other studies where the mean duration of tumour resolution was considered 3-4 weeks [30-32]. Recurrences take place most frequently within the first 6 months after resection [2]. The patients were followed up for 6 months post treatment and no recurrence was noted in our study.
Hirst, in the only randomized clinical trial to assess the effectiveness of MMC 0.004% as primary topical treatment in OSSN, found that 93% of the patients had complete resolution with topical MMC. There were no severe side effects [4]. In a retrospective study done by Kusumesh et al complete response was achieved in 92% treated with topical MMC 0.004% in a median duration of 1.5 months. Adverse reaction was noted in 88% of the patients. Most common adverse effect was conjunctival hyperemia [22]. All patients achieved complete response after one month of treatment, however, the rate of recurrence was 4.3% after 24 months of treatment. Corneal erosion was noted in 17.4% of the patients [9]. Rozenman and Frucht-Pery found MMC both in 0.02% and 0.04% were effective as primary treatment for small-size conjunctival intraepithelial neoplasia (CIN). No serious adverse reactions to MMC treatment were found except conjunctival hyperemia with irritation which disappeared on cessation of treatment [29]. Shields CL and associates in their prospective studies on MMC for OSSN (both primary and recurrent) found that topical mitomycin C 0.04% is safe and effective therapy for conjunctival or corneal SCC [31, 32].
The limitations of the study were smaller sample size, non-randomized treatment algorithm, and shorter duration of follow-up.
Conclusion
The study shows that topical chemotherapy in the form of MMC 0.02% used in pulsed manner is effective in treatment of OSSN with negligible side effects. Absence of progression of tumour during the treatment and presence of response in the form of complete resolution or partial resolution leading, to chemoreduction in all patients in our study support the use of topical chemotherapeutic agents as primary treatment. Hence a trial of topical treatment may be used in all suspected cases of OSSN before doing surgical excision. Considering its safety and efficacy, MMC has the potential to be chosen as the first line agent in the topical treatment of OSSN.
Conflict of interest
Authors have no conflicts of interest to disclose.
References
[1] Adler E, Turner JR, Stone DU. Ocular surface squamous neoplasia: a survey of changes in the standard of care from 2003 to 2012. Cornea. 2013; 32(12):1558–1561.
[2] Cicinelli MV, Marchese A, Bandello F, Modorati G. Clinical Management of Ocular Surface Squamous Neoplasia: A Review of the Current Evidence. Ophthalmol Ther. 2018; 7(2):247–262.
[3] Honavar SG. Ocular surface squamous neoplasia: Are we calling a spade a spade?. Indian J Ophthalmol. 2017; 65:907–909.
[4] Hirst LW. Randomized controlled trial of topical mitomycin C for ocular surface squamous neoplasia: early resolution. Ophthalmology. 2007; 114(5):976–982.
[5] Ma IH, Hu FR, Wang IJ, Chen WL, Hsu YJ, et al. Clinicopathologic correlation of ocular surface squamous neoplasia from a university hospital in North Taiwan 1994 to 2014. J Formos Med Assoc. 2019; 118(4):776–782.
[6] Parrozzani R, Frizziero L, Trainiti S, Tetsi I, Miglionico G, et al. Topical 1% 5-fluoruracil as a sole treatment of corneoconjunctival ocular surface squamous neoplasia: long-term study. Br J Ophthalmol. 2017; 101(8):1094–1099.
[7] Stone DU, Butt AL, Chodosh J. Ocular surface squamous neoplasia: a standard of care survey. Cornea. 2005; 24(3):297–300.
[8] Meel R, Dhiman R, Vanathi M, Pushker N, Tandon R, et al. Clinicodemographic profile and treatment outcome in patients of ocular surface squamous neoplasia. Indian J Ophthalmol. 2017; 65(10):936–941.
[9] Honavar SG, Manjandavida FP. Tumors of the ocular surface: A review. Indian J Ophthalmol 2015; 63(3):187–203.
[10] Carreira H, Coutinho F, Carrilho C, Lunet N. HIV and HPV infections and ocular surface squamous neoplasia: systematic review and meta-analysis. Br J Cancer. 2013; 109(7):1981–1988.
[11] Gichuhi S, Sagoo MS, Weiss HA, Burton MJ. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013; 18(12):1424–1443.
[12] Galor A, Karp CL, Oellers P, Kao AA, Abdelaziz A, et al. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. 2012; 119(10):1974–1981.
[13] Gupta A, Muecke J. Treatment of ocular surface squamous neoplasia with mitomycin C. Br J Ophthalmol. 2010; 94(5):555–558.
[14] Kim JW, Abramson DH. Topical treatment options for conjunctival neoplasms. Clin Ophthalmol. 2008; 2(3):503–515.
[15] Viani GA, Fendi LI. Adjuvant treatment or primary topical monotherapy for ocular surface squamous neoplasia: a systematic review. Arq Bras Oftalmol. 2017; 80(2):131–136.
[16] Khong JJ, Muecke J. Complications of mitomycin C therapy in 100 eyes with ocular surface neoplasia. Br J Ophthalmol. 2006; 90(7):819–822.
[17] Gupta S, Sinha R, Sharma N, Titiyal JS. Ocular surface squamous neoplasia. 2012; 23(2):90–96.
[18] Taylor R, Aronow MB, Correa ZM, Demicri H. OSSN: Trends in topical chemotherapy. AAO. Eye Net Magazine. Accessed on 30 August. 2018, from: https://www.aao.org/eyenet/article/ossn-trends-in-topical-chemotherapy
[19] Jaber R, Karp CL. Topical chemotherapy for ocular surface squamous neoplasia. AAO. Eye Net Magazine. Accessed on 27 March 2012 from: https://www.aao.org/eyenet/article/topical-chemotherapy-ocular-surface-squamous-neopl
[20] Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000; 118(7):885–891.
[21] Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010; 94(10):1316–1321.
[22] Kusumesh R, Ambastha A, Kumar S, Sinha BP, Imam N. Retrospective comparative study of topical interferon α2b versus mitomycin C for primary ocular surface squamous neoplasia. Cornea. 2017; 36(3):327–331.
[23] McKelvie PA, Daniell M. Impression cytology following mitomycin C therapy for ocular surface squamous neoplasia. Br J Ophthalmol. 2001; 85(9):1115–1119.
[24] McClellan AJ, McClellan AL, Pezon CF, Karp CL, Feuer W, et al. Epidemiology of ocular surface squamous neoplasia in a veterans affairs population. Cornea. 2013; 32(10):13548.
[25] Kim BH, Kim MK, Wee WR, Oh JY. Clinical and pathological characteristics of ocular surface squamous neoplasia in an Asian population. Graefes Arch Clin Exp Ophthalmol. 2013; 251(11):2569–2573.
[26] Al Bayyat G, Arreaza-Kaufman D, Venkateswaran N, Galor A, Karp CL. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis (Lond). 2019; 6:24.
[27] Kusumesh R, Ambastha A, Kumar S, Sinha BP, Imam N. Retrospective comparative study of topical interferon alpha2b versus mitomycin c for primary ocular surface squamous neoplasia. Cornea. 2017; 36(3):327–331.
[28] Poothullil AM, Colby KA. Topical medical therapies for ocular surface tumors. Semin Ophthalmol. 2006; 21(3):161–169.
[29] Rozenman Y, Frucht-Pery J. Treatment of conjunctival intraepithelial neoplasia with topical drops of mitomycin C. Cornea. 2000; 19(1):1–6.
[30] Sayed-Ahmed IO, Palioura S, Galor A, Karp CL. Diagnosis and medical management of ocular surface squamous neoplasia. Expert Rev Ophthalmol. 2017; 12(1):11–19.
[31] Shields CL, Naseripour M, Shields JA. Topical mitomycin C for extensive, recurrent conjunctival-corneal squamous cell carcinoma. Am J Ophthalmol. 2002; 133(5):601–606.
[32] Shields CL, Demirci H, Marr BP, Masheyekhi A, Materin M, Shields JA. Chemoreduction with topical mitomycin C prior to resection of extensive squamous cell carcinoma of the conjunctiva. Arch Ophthalmol. 2005; 123(1):109–113.